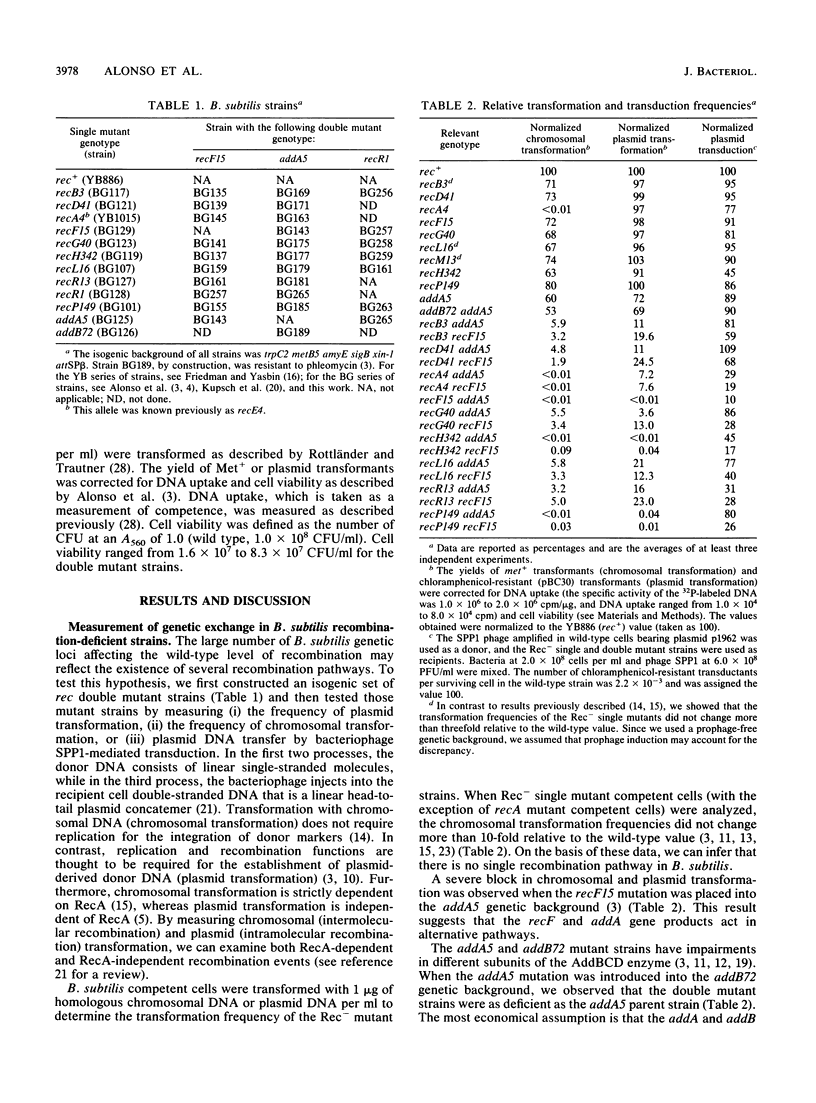
Abstract
Recombination in Bacillus subtilis requires the products of numerous rec loci. To dissect the various mechanisms which may be involved in genetic recombination, we constructed a series of isogenic strains containing more than one mutant rec allele. On the basis of their impairment in genetic exchange, the various loci (represented by specific rec alleles) were classified into different epistatic groups. Group alpha consists of rec genes represented by recB, recD, recF, recG, recL, and recR mutations, while group beta comprises the addA and addB mutations. Group gamma consists of the recH and recP mutations. These results suggest that B. subtilis has multiple pathways for genetic recombination and that the products of the genes within the alpha, beta, and gamma epistatic groups are involved in these alternative recombination pathways. The RecA protein is required in all three pathways of intermolecular recombination.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso J. C., Shirahige K., Ogasawara N. Molecular cloning, genetic characterization and DNA sequence analysis of the recM region of Bacillus subtilis. Nucleic Acids Res. 1990 Dec 11;18(23):6771–6777. doi: 10.1093/nar/18.23.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso J. C., Tailor R. H., Lüder G. Characterization of recombination-deficient mutants of Bacillus subtilis. J Bacteriol. 1988 Jul;170(7):3001–3007. doi: 10.1128/jb.170.7.3001-3007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso J. C., Viret J. F., Tailor R. H. Plasmid maintenance in Bacillus subtilis recombination-deficient mutants. Mol Gen Genet. 1987 Jun;208(1-2):349–352. doi: 10.1007/BF00330464. [DOI] [PubMed] [Google Scholar]
- Canosi U., Morelli G., Trautner T. A. The relationship between molecular structure and transformation efficiency of some S. aureus plasmids isolated from B. subtilis. Mol Gen Genet. 1978 Nov 9;166(3):259–267. doi: 10.1007/BF00267617. [DOI] [PubMed] [Google Scholar]
- Ceglowski P., Lüder G., Alonso J. C. Genetic analysis of rec E activities in Bacillus subtilis. Mol Gen Genet. 1990 Jul;222(2-3):441–445. doi: 10.1007/BF00633853. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Sandler S. J., Willis D. K., Chu C. C., Blanar M. A., Lovett S. T. Genes of the RecE and RecF pathways of conjugational recombination in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1984;49:453–462. doi: 10.1101/sqb.1984.049.01.051. [DOI] [PubMed] [Google Scholar]
- Deichelbohrer I., Alonso J. C., Lüder G., Trautner T. A. Plasmid transduction by Bacillus subtilis bacteriophage SPP1: effects of DNA homology between plasmid and bacteriophage. J Bacteriol. 1985 Jun;162(3):1238–1243. doi: 10.1128/jb.162.3.1238-1243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doly J., Le Roscouët D., Anagnostopoulos C. Substrate specificity and adenosine triphosphatase activity of the ATP-dependent deoxyribonuclease of Bacillus subtilis. Eur J Biochem. 1981 Mar;114(3):493–499. doi: 10.1111/j.1432-1033.1981.tb05172.x. [DOI] [PubMed] [Google Scholar]
- Doly J., Sasarman E., Anagnostopoulos C. ATP-dependent deoxyribonuclease in Bacillus subtilis and a mutant deficient in this activity. Mutat Res. 1974 Jan;22(1):15–23. doi: 10.1016/0027-5107(74)90003-7. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: nonrequirement of deoxyribonucleic acid replication for uptake and integration of transforming deoxyribonucleic acid. J Bacteriol. 1973 Mar;113(3):1512–1514. doi: 10.1128/jb.113.3.1512-1514.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Scher B., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: phenotypic characterization of radiation-sensitive recombination-deficient mutants. J Bacteriol. 1973 Apr;114(1):273–286. doi: 10.1128/jb.114.1.273-286.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B. M., Yasbin R. E. The genetics and specificity of the constitutive excision repair system of Bacillus subtilis. Mol Gen Genet. 1983;190(3):481–486. doi: 10.1007/BF00331080. [DOI] [PubMed] [Google Scholar]
- Gassel M., Alonso J. C. Expression of the recE gene during induction of the SOS response in Bacillus subtilis recombination-deficient strains. Mol Microbiol. 1989 Sep;3(9):1269–1276. doi: 10.1111/j.1365-2958.1989.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Kooistra J., Vosman B., Venema G. Cloning and characterization of a Bacillus subtilis transcription unit involved in ATP-dependent DNase synthesis. J Bacteriol. 1988 Oct;170(10):4791–4797. doi: 10.1128/jb.170.10.4791-4797.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupsch J., Alonso J. C., Trautner T. A. Analysis of structural and biological parameters affecting plasmid deletion formation in Bacillus subtilis. Mol Gen Genet. 1989 Sep;218(3):402–408. doi: 10.1007/BF00332402. [DOI] [PubMed] [Google Scholar]
- Love P. E., Yasbin R. E. Genetic characterization of the inducible SOS-like system of Bacillus subtilis. J Bacteriol. 1984 Dec;160(3):910–920. doi: 10.1128/jb.160.3.910-920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett C. M., Jr, Roberts J. W. Purification of a RecA protein analogue from Bacillus subtilis. J Biol Chem. 1985 Mar 25;260(6):3305–3313. [PubMed] [Google Scholar]
- Mazza P., Galizzi A. Revised genetics of DNA metabolism in Bacillus subtilis. Microbiologica. 1989 Apr;12(2):157–179. [PubMed] [Google Scholar]
- Ogasawara N., Moriya S., von Meyenburg K., Hansen F. G., Yoshikawa H. Conservation of genes and their organization in the chromosomal replication origin region of Bacillus subtilis and Escherichia coli. EMBO J. 1985 Dec 1;4(12):3345–3350. doi: 10.1002/j.1460-2075.1985.tb04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi S., Sueoka N. Adenosine triphosphate-dependent deoxyribonuclease in Bacillus subtilis. J Biol Chem. 1973 Nov 10;248(21):7336–7344. [PubMed] [Google Scholar]
- Rottländer E., Trautner T. A. Genetic and transfection studies with B, subtilis phage SP 50. I. Phage mutants with restricted growth on B. subtilis strain 168. Mol Gen Genet. 1970;108(1):47–60. doi: 10.1007/BF00343184. [DOI] [PubMed] [Google Scholar]
- Shemyakin M. F., Grepachevsky A. A., Chestukhin A. V. Properties of Bacillus subtilis ATP-dependent deoxyribonuclease. Eur J Biochem. 1979 Aug 1;98(2):417–423. doi: 10.1111/j.1432-1033.1979.tb13201.x. [DOI] [PubMed] [Google Scholar]
- Smith G. R. Homologous recombination in E. coli: multiple pathways for multiple reasons. Cell. 1989 Sep 8;58(5):807–809. doi: 10.1016/0092-8674(89)90929-x. [DOI] [PubMed] [Google Scholar]
- Stranathan M. C., Bayles K. W., Yasbin R. E. The nucleotide sequence of the recE+ gene of Bacillus subtilis. Nucleic Acids Res. 1990 Jul 25;18(14):4249–4249. doi: 10.1093/nar/18.14.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos W. M., Venema G., Canosi U., Trautner T. A. Plasmid transformation in Bacillus subtilis: fate of plasmid DNA. Mol Gen Genet. 1981;181(4):424–433. doi: 10.1007/BF00428731. [DOI] [PubMed] [Google Scholar]


