Abstract
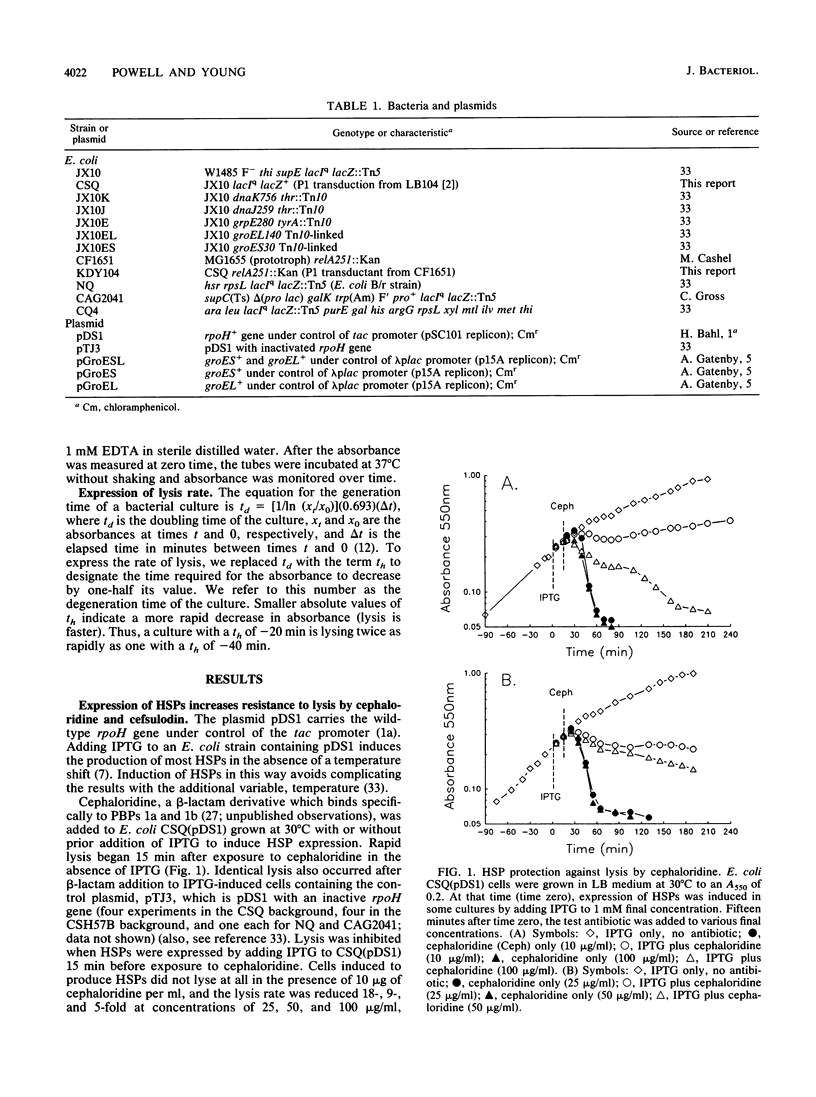
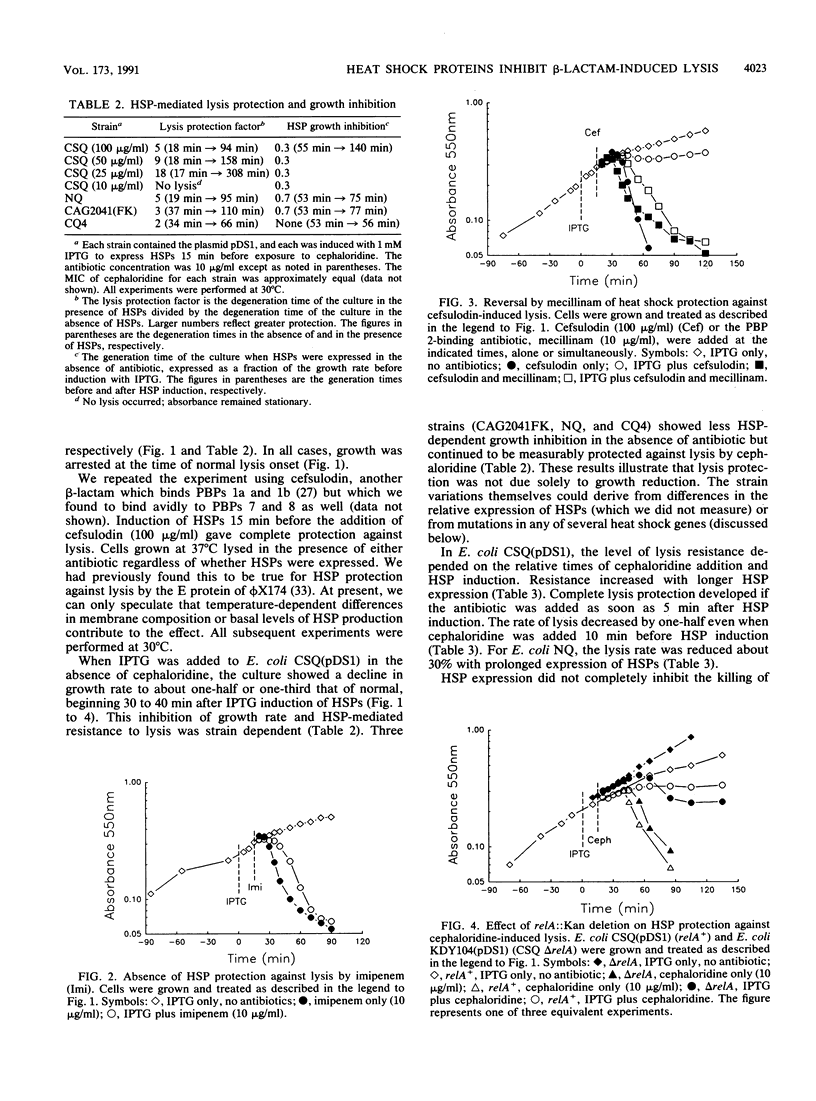
The heat shock proteins (HSPs) of Escherichia coli were artificially induced in cells containing the wild-type rpoH+ gene under control of a tac promoter. At 30 degrees C, expression of HSPs produced cells that were resistant to lysis by cephaloridine and cefsulodin, antibiotics that bind penicillin-binding proteins (PBPs) 1a and 1b. This resistance could be reversed by the simultaneous addition of mecillinam, a beta-lactam that binds PBP 2. However, even in the presence of mecillinam, cells induced to produce HSPs were resistant to lysis by ampicillin, which binds all the major PBPs. Lysis of cells induced to produce HSPs could also be effected by imipenem, a beta-lactam known to lyse nongrowing cells. These effects suggest the existence of at least two pathways for beta-lactam-dependent lysis, one inhibited by HSPs and one not. HSP-mediated lysis resistance was abolished by a mutation in any one of five heat shock genes (dnaK, dnaJ, grpE, GroES, or groEL). Thus, resistance appeared to depend on the expression of the complete heat shock response rather than on any single HSP. Resistance to lysis was significant in the absence of the RelA protein, implying that resistance could not be explained by activation of the stringent response. Since many environmental stresses promote the expression of HSPs, it is possible that their presence contributes an additional mechanism toward development in bacteria of phenotypic tolerance to beta-lactam antibiotics.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahl H., Echols H., Straus D. B., Court D., Crowl R., Georgopoulos C. P. Induction of the heat shock response of E. coli through stabilization of sigma 32 by the phage lambda cIII protein. Genes Dev. 1987 Mar;1(1):57–64. doi: 10.1101/gad.1.1.57. [DOI] [PubMed] [Google Scholar]
- Breeden L., Yarus M., Cline S. A cloned suppressor tRNA gene relaxes stringent control. Mol Gen Genet. 1980;179(1):125–133. doi: 10.1007/BF00268454. [DOI] [PubMed] [Google Scholar]
- Cozens R. M., Markiewicz Z., Tuomanen E. Role of autolysins in the activities of imipenem and CGP 31608, a novel penem, against slowly growing bacteria. Antimicrob Agents Chemother. 1989 Oct;33(10):1819–1821. doi: 10.1128/aac.33.10.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Goodell W., Tomasz A. Alteration of Escherichia coli murein during amino acid starvation. J Bacteriol. 1980 Dec;144(3):1009–1016. doi: 10.1128/jb.144.3.1009-1016.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A. D., Straus D. B., Walter W. A., Gross C. A. Sigma 32 synthesis can regulate the synthesis of heat shock proteins in Escherichia coli. Genes Dev. 1987 Apr;1(2):179–184. doi: 10.1101/gad.1.2.179. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Taylor W. E., Burton Z. F., Burgess R. R., Gross C. A. Stringent response in Escherichia coli induces expression of heat shock proteins. J Mol Biol. 1985 Nov 20;186(2):357–365. doi: 10.1016/0022-2836(85)90110-x. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Vincent S., Billot-Klein D., Acar J. F., Mrèna E., Williamson R. Involvement of penicillin-binding protein 2 with other penicillin-binding proteins in lysis of Escherichia coli by some beta-lactam antibiotics alone and in synergistic lytic effect of amdinocillin (mecillinam). Antimicrob Agents Chemother. 1986 Dec;30(6):906–912. doi: 10.1128/aac.30.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusser W., Ishiguro E. E. Lysis of nongrowing Escherichia coli by combinations of beta-lactam antibiotics and inhibitors of ribosome function. Antimicrob Agents Chemother. 1986 Mar;29(3):451–455. doi: 10.1128/aac.29.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusser W., Ishiguro E. E. Suppression of mutations conferring penicillin tolerance by interference with the stringent control mechanism of Escherichia coli. J Bacteriol. 1987 Sep;169(9):4396–4398. doi: 10.1128/jb.169.9.4396-4398.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusser W., Pisabarro A. G., De Pedro M. A., Ishiguro E. E. Decay of the ampicillin-induced lysis process in amino acid-deprived Escherichia coli. Antimicrob Agents Chemother. 1990 Jan;34(1):164–166. doi: 10.1128/aac.34.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc M., Kasra R., van Heijenoort J. Induction and control of the autolytic system of Escherichia coli. J Bacteriol. 1982 Oct;152(1):26–34. doi: 10.1128/jb.152.1.26-34.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc M., van Heijenoort J. Autolysis of Escherichia coli. J Bacteriol. 1980 Apr;142(1):52–59. doi: 10.1128/jb.142.1.52-59.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger S., Schreiber G., Aizenman E., Cashel M., Glaser G. Characterization of the relA1 mutation and a comparison of relA1 with new relA null alleles in Escherichia coli. J Biol Chem. 1989 Dec 15;264(35):21146–21152. [PubMed] [Google Scholar]
- Neu J. C. Synergy of mecillinam, a beta-amidinopenicillanic acid derivative, combined with beta-lactam antibiotics. Antimicrob Agents Chemother. 1976 Sep;10(3):535–542. doi: 10.1128/aac.10.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd K. E., Bochner B. R., Cashel M., Roth J. R. Mutations in the spoT gene of Salmonella typhimurium: effects on his operon expression. J Bacteriol. 1985 Aug;163(2):534–542. doi: 10.1128/jb.163.2.534-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Straus D. B., Walter W. A., Gross C. A. Escherichia coli heat shock gene mutants are defective in proteolysis. Genes Dev. 1988 Dec;2(12B):1851–1858. doi: 10.1101/gad.2.12b.1851. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Penicillin-binding proteins and the antibacterial effectiveness of beta-lactam antibiotics. Rev Infect Dis. 1986 Jul-Aug;8 (Suppl 3):S260–S278. doi: 10.1093/clinids/8.supplement_3.s260. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Cozens R., Tosch W., Zak O., Tomasz A. The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J Gen Microbiol. 1986 May;132(5):1297–1304. doi: 10.1099/00221287-132-5-1297. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Markiewicz Z., Tomasz A. Autolysis-resistant peptidoglycan of anomalous composition in amino-acid-starved Escherichia coli. J Bacteriol. 1988 Mar;170(3):1373–1376. doi: 10.1128/jb.170.3.1373-1376.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E. Phenotypic tolerance: the search for beta-lactam antibiotics that kill nongrowing bacteria. Rev Infect Dis. 1986 Jul-Aug;8 (Suppl 3):S279–S291. doi: 10.1093/clinids/8.supplement_3.s279. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Tomasz A. Induction of autolysis in nongrowing Escherichia coli. J Bacteriol. 1986 Sep;167(3):1077–1080. doi: 10.1128/jb.167.3.1077-1080.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- Young K. D., Anderson R. J., Hafner R. J. Lysis of Escherichia coli by the bacteriophage phi X174 E protein: inhibition of lysis by heat shock proteins. J Bacteriol. 1989 Aug;171(8):4334–4341. doi: 10.1128/jb.171.8.4334-4341.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


