Abstract
UV radiation induces immunosuppression and inflammatory responses, as well as oxidative stress and DNA damage, in skin cells and these various effects have been implicated in melanoma and nonmelanoma skin cancers, i.e., photocarcinogenesis. The cytokine interleukin (IL)-12 has been shown to possess potent anti-tumor activity in a wide variety of murine tumor models. In this review, we summarize the evidence that IL-12 plays a role in preventing photocarcinogenesis, and present a model of its possible mechanisms of action. Treatment of mice with IL-12 prevents UV-induced immunosuppression in a process mediated by repair of UV-induced damaged DNA. After exposure to the photocarcinogenesis protocol, the development of UV-induced tumors is more rapid and the tumor multiplicity and tumor size are significantly greater in IL-12-deficient or knockout (KO) mice than their wild-type counterparts. IL-12-deficiency in mice enhances the proliferation potential of tumor cells, and this may be one of the reasons for the rapid growth of the tumors and their greater size. The rate of malignant transformation of UV-induced papillomas to carcinomas also is higher in the IL-12 KO mice than in their wild-type counterparts in terms of carcinoma incidence and carcinoma multiplicity. UV-induced DNA damage in the form of cyclobutane pyrimidine dimers (CPDs) and sunburn cells is lower, or repaired more rapidly, in wild-type mice than IL-12 KO mice. The IL-12-associated reduction in UV-specific CPDs is due to induction of DNA repair, and particularly enhancement of nucleotide-excision repair. We suggest that endogenous stimulation of IL-12 may protect the skin from UV-induced immunosuppression, DNA damage, and, ultimately, the risk of photocarcinogenesis. Taken together, these information suggest that augmentation of IL-12 should be considered as a strategy for the prevention and treatment of photocarcinogenesis.
Keywords: Interleukin-12, photocarcinogenesis, cyclobutane pyrimidine dimers, UV radiation
Introduction
Interleukin (IL)-12 is a 70-kDa heterodimeric protein composed of two disulfide-bonded chains, the p40 and p35 subunits (Wolf et al., 1991; Kobayashi et al., 1989). Only the heterodimer shows biologic activity, although a possible inhibitory function of the IL-12p40 chain homodimer has been reported (Mattner et al., 1993). IL-12 is produced principally by antigen presenting cells, such as dendritic cells, monocytes and macrophages. Initially, IL-12 was identified as natural killer cell stimulatory factor (Wolf et al., 1991); however, it also activates cytotoxic T cells (Mattner et al., 1993; Trinchieri, 1994), stimulates differentiation of CD4+ lymphocytes (Hayes et al., 1995; Trinchieri and Scott, 1995), plays an important role in regulating the balance between type 1 and type 2 responses of Th lymphocytes (Manetti et al., 1993; Trinchiery, 1993), and possesses IFN-γ-dependent anti-angiogenic activity (Voest et al, 1995; Yao et al., 1999).
IL-12 has been shown to possess potent anti-tumor activity in a wide variety of murine tumor models (Colombo et al., 1996; Brunda et al., 1993; Zou et al., 1995; Robertson and Ritz, 1996) including carcinomas from the colon, kidney, and lung (Nastala et al., 1994; Wigginton et al., 1996; Tahara et al., 1994; Mu et al., 1995) with the presence of IL-12 at the tumor site being critical for tumor regression (Colombo et al., 1996). Conversely, the tumor-bearing state is often characterized by a diminished capacity to produce IL-12 and an increase in the levels of IL-10 (Halak et al., 1999). The demonstration of significant antitumor activity in several preclinical animal tumor models has stimulated interest in the therapeutic use of IL-12 (Trinchiery, 1994; Chen et al., 1997; Siders et al., 1998) but the mechanisms underlying the antitumor activity of IL-12 are not clearly understood. In the present review article, we discuss the role of IL-12 in protection against adverse biological effects induced by UV irradiation, which in combination contribute to the development of nonmelanoma skin cancers or photocarcinogenesis.
Characteristic of UV radiation
Although several environmental and genetic factors contribute to the development of skin cancers, chronic exposure to solar ultraviolet (UV) radiation is the main etiologic factor for this disease. The UV radiation present in sun light is generally divided into three regions, short-wave (UVC; 200-290 nm), mid-wave (UVB; 290-320 nm), and long-wave (UVA; 320-400 nm) (Katiyar, 2006). UVC is a potent mutagen and can induce immune suppression; however, it is absorbed efficiently by the stratospheric ozone layer, so its role in human pathogenesis is minimal. Reduction in stratospheric ozone layer allows an increase in the levels of UVB radiation that reach the Earth’s surface (Van der Leun, 1989). Solar UVB radiation can act as a tumor initiator, tumor promoter (Katiyar et al., 1997) and co-carcinogen (Donawho and Kripke, 1991; Ziegler et al., 1994). Exposure of skin to UVB radiation results in a variety of biologic effects including inflammation, induction of oxidative stress, formation of sunburn cells, DNA damage, and immunologic alterations, all of which play important roles in the development of melanoma and nonmelanoma skin cancers (Donawho and Kripke, 1991; Ziegler et al., 1994; Katiyar and Mukhtar, 2001). UVB radiation has multiple effects on the immune system (Meunier et al., 1998) resulting in adverse effects on human health including exacerbation of infectious diseases and greater risk of skin cancer (Urbach, 1991; Chapman et al., 1995). UVB-induced immune suppression is considered as a risk factor for the development of skin cancer (Donawho and Kripke, 1991; Ziegler et al., 1994; Yoshikawa et al., 1990). It has been recognized that chronically immunosuppressed patients living in regions of intense sun exposure experience an exceptionally high rate of skin cancer particularly in sun-exposed areas (Kinlen et al., 1979). In addition, an increased incidence of skin cancers, especially squamous cell carcinomas, has been noted among recipients of organ transplants (Cowen and Billingsley, 1999; Otley and Pittelkow, 2000; Fortina et al., 2000). This increased incidence of squamous cell carcinomas in transplant patients is presumably attributable to long-term immunosuppressive therapy (DiGiovanna, 1998), although nonimmune mechanisms also may play a role. Thus, a considerable body of evidence implicates UVB-induced immunosuppression in the development of melanoma and non-melanoma skin cancers. UVA, the major component of the UV portion of the solar spectrum, also causes premature aging of the skin, induce oxidative stress and can suppress some immunologic functions but its effect is less potent than UVB (Ullrich, 1995; Mukhtar and Elmets, 1996).
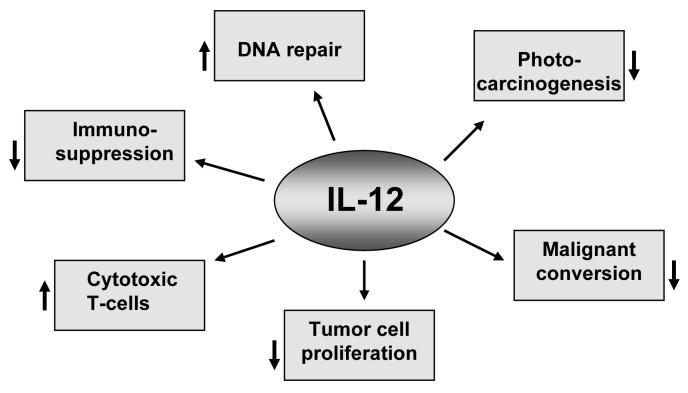
UV-induced skin tumors in mice are highly immunogenic and are rejected when transplanted into naïve syngeneic hosts (Romerdahl et al., 1989). The ability of the injected tumors to grow in recipient mice treated with immunosuppressive drugs further indicates that the rejection is immunologic in nature. Tumor rejection also is prevented when the recipient mice are exposed to UVB radiation (Schwarz, 2005), clearly indicating that UV radiation can act in a manner similar to immunosuppressive drugs. As it has been reported that IL-12 has the ability to reverse UVB-induced immunosuppression and possesses anti-tumor activity, we are investigating its role in inhibition of photocarcinogenesis. The major targets and events affected by IL-12 in UV-exposed skin have been summarized in Fig. 1.
Fig. 1.
Molecular targets or events generally affected by IL-12 in UVB-exposed skin are summarized. Downward arrows (↓) indicate the inhibition or suppression, and upward arrows indicate the enhancement or stimulation (↑).
IL-12 prevents UV-induced immune suppression
Exposure of murine skin to UV radiation suppresses the development of allergic contact hypersensitivity (CHS), a prototypic T-cell mediated immune response (Kripke, 1990). IL-12 has been shown to stimulate T-cell mediated immune responses. Consistent with its role as a cytokine that stimulates cell-mediated immune reactions, IL-12 has been shown to reverse the immunosuppressive effects of UVB-radiation on CHS responses. For example, injection of anti-IL-12 mAb before allergen painting prevents sensitization in vivo, whereas administration of IL-12 breaks UV-induced tolerance in mice, thus demonstrating the important adjuvant function of this cytokine (Muller et al., 1995; Schmitt et al., 1995). Intraperitoneal injection of murine rIL-12 prevents UV-induced local immunosuppression and overcomes UV-induced hapten-specific tolerance (Schwarz et al., 1996). The mice that were initially sensitized through UV-exposed skin also produced a significant CHS reaction when they received rIL-12 before resensitization. In chemoprevention studies, it has been shown that dietary feeding of grape seed proanthocyanidins inhibit UVB-induced immunosuppression, and the inhibition of immunosuppression by grape seed proanthocyanidins was associated with the induction of IL-12 in mice (Sharma and Katiyar, 2006). Similarly, it was also found that topical treatment of mice with silymarin, a plant flavonoid, inhibits UVB-induced immunosuppression through augmentation of IL-12 in the skin and draining lymph nodes (Meeran et al., 2006). These observations support the notion that IL-12 has a significant preventive role in UV radiation-induced immune suppression. Because immune suppression is considered as a risk factor for cancer induction (Meunier et al., 1998; Yoshikawa et al., 1990), IL-12 may have a role in the prevention of UV radiation-induced skin cancer.
IL-12 prevents UV-induced immunosuppression through DNA repair mechanism
Mechanistic studies were performed to examine the role of IL-12 as an adjuvant to UVB-induced immunosuppression using in vitro and in vivo animal models. UV-induced DNA damage, predominantly in the form of cyclobutane pyrimidine dimers (CPDs) or thymine dimers, has been recognized as an important molecular trigger for the suppression of immune responses (Applegate et al., 1989) and initiation of UV-carcinogenesis (Applegate et al., 1989; Kripke et al., 1992; Yarosh et al., 1992). In turn, accelerated or enhanced removal of UV-induced CPDs through the topical application of the bacterial DNA repair enzyme, T4 endonuclease V, impaired the development of skin tumors in mice exposed to chronic UV radiation (Yarosh et al., 1992). Recently, we and others have reported that IL-12 exhibits the capacity to remove or repair UV-induced DNA damage, and that the prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. This repair of damaged DNA by IL-12 is mediated through nucleotide excision repair mechanism (Schwarz et al., 2002; Meeran et al., 2006). If the DNA damage is irreparable, the keratinocyte cell cycle is arrested and it is transformed into a sunburn cell, an initial morphological indicator of epidermal cell apoptosis.
To further confirm the role of IL-12 in removal or repair of UVB-induced damaged DNA, IL-12-knockout (KO) or -deficient mice and their wild-type, C3H/HeN, mice were employed. Mice were exposed to UVB radiation, then sacrificed immediately or 24 hours later. Skin samples were collected and subjected to immunohistochemical detection of CPD-positive cells using an antibody directed against CPDs. In skin samples obtained immediately after UV exposure, no differences in the staining pattern of CPDs were observed between IL-12 KO and wild-type mice. However, in samples obtained 24 hours after UVB exposure, the numbers of CPD-positive cells were significantly lower in the wild-type mice compared to the number of CPD-positive cells obtained immediately after UV exposure. In contrast, the number of CPD-positive cells in IL-12 KO mice 24 hours after UVB exposure was not significantly lower than the number detected immediately after UV exposure (Meeran et al., 2006a; Meeran et al., 2006b; Meeran et al., 2006c; Maeda et al., 2006). Moreover, the spontaneous DNA repair was greater in wild-type mice than IL-12 KO mice. This suggests that the difference in DNA repair between wild-type and IL-12 KO mice may be due to the absence of IL-12 in the IL-12 KO mice. Identical information also was observed when epidermal genomic DNA from UVB-exposed wild-type and IL-12 KO mice were examined and compared using dot-blot analysis. In this case, the intensity of the dot-blot indicating the presence of genomic DNA containing CPDs, of samples obtained from wild-type mice 24 hours after UV exposure was significantly lower than of samples obtained from wild-type mice immediately after UV exposure. In contrast, the intensity of the dot-blot of samples obtained from IL-12 KO mice 24 hours after UV exposure was not significantly lower than that of samples obtained immediately after UV exposure (Meeran et al., 2006c; Maeda et al., 2006). These observations further support the evidence that wild-type mice are able to rapidly remove or repair UV-induced DNA damage in the form of CPDs whereas IL-12 KO mice are not. This difference may be due to the presence of IL-12 in wild-type mice or absence of IL-12 in IL-12 KO mice.
It has been recognized that UV-induced DNA damage is an important molecular trigger for the migration of antigen presenting cells (i.e., Langerhans cells in the epidermis) from the skin to the draining lymph nodes. DNA damage in antigen presenting cells impairs their capacity to present antigen, which in turn results in a lack of sensitization (Vink et al., 1997). CPD-containing antigen presenting cells have been found in the draining lymph nodes of UV-exposed mice (Vink et al., 1996). These antigen presenting cells were identified to be of epidermal origin and exhibited an impaired antigen presentation capacity. This phenomenon was examined and compared in the IL-12 KO and their wild-type counterparts. Mice were exposed to UVB radiation, sacrificed 24 hours later and the presence of CPD-positive cells in draining lymph nodes samples detected using immunohistochemical analysis. Significant numbers of CPD-positive cells in the draining lymph nodes were found in both UV-exposed wild-types and IL-12 KO mice, with the numbers of CPD-positive cells in the draining lymph nodes of the UV-exposed IL-12 KO mice being approximately four-fold higher than in the draining lymph nodes of their wild-type counterparts. The lower percentage of CPD-positive cell in the draining lymph nodes of UV-exposed wild-type mice compared to IL-12 KO mice may be attributable to the presence of endogenous IL-12 in the wild-type mice at levels that are capable of partial removal of the damaged DNA in the migrating cells. There is evidence that UV-induced DNA damage is the molecular trigger for the migration of the Langerhans cells, which are the antigen presenting cells in the epidermis, from the skin to the draining lymph nodes. The UV-induced DNA damage also impairs the antigen presenting capacity of Langerhans cells which results in a lack of sensitization and the induction of tolerance to contact sensitizers. Similar observations were observed when xeroderma pigmentosum complementation group A-deficient (XPA-deficient) and their wild-type counterparts were subjected to a contact hypersensitivity protocol (Schwarz et al., 2005). XPA-deficient mice are devoid of nucleotide excision repair mechanisms, and therefore unable to repair UVB-induced DNA damage. Schwarz et al. (2005) have demonstrated that treatment of IL-12 prevents both UV-induced suppression of the induction of contact hypersensitivity and the depletion of Langerhans cells in wild-type but not in DNA-repair-deficient (XPA) mice. As UV-induced DNA damage and immunosuppression play an important role in melanoma and nonmelanoma skin cancers, it is tempting to suggest that IL-12 should be tested as a therapeutic agent for the prevention of skin cancers in humans.
IL-12-deficiency enhances photocarcinogenesis
To define the role of IL-12 in photocarcinogenesis, IL-12 KO mice (IL-12p35-/- or IL-12p40-/-) and their wild-type counterparts were subjected to a photocarcinogenesis protocol (Meeran et al., 2006c; Maeda et al., 2006). The tumor incidence (percentage of mice with tumors), tumor multiplicity (total number of tumors/group) and tumor size (mean tumor size in mm3/tumor in a group of 20 mice) were recorded on weekly basis. The results demonstrated that the appearance of tumors occurred earlier, the tumor multiplicity was higher, and the tumor growth was faster, in IL-12 KO mice than wild-type mice. Throughout the photocarcinogenesis protocol, the percentage of IL-12 KO mice with tumors was higher than the percentage of wild-type mice with tumors. In terms of tumor multiplicity, the total number of tumors in the group of IL-12 KO mice remained higher than the total number in the group of wild-type mice throughout the experimental protocol. At the termination of the experiment at 35 weeks, the total number of tumors was significantly higher in the group of IL-12 KO mice than the group of wild-type mice (72 vs. 42, p<0.01). In terms of tumors per tumor bearing mouse, a higher number of tumors per tumor bearing mouse was observed throughout the experiment in the group of IL-12 KO mice than in the group of wild-type mice and was significantly higher at the termination of the experiment (p<0.05). Similarly, the tumor volume per tumor was significantly higher in the group of IL-12 KO mice compared to their wild-types. Taken together, these observations indicate that IL-12-deficient mice are susceptible to UVB-induced carcinogenesis.
IL-12-deficiency inhibits and prolongs the repair of UVB-induced DNA damage
UVB-induced CPDs have been implicated in initiation of photocarcinogenesis in mice. We have observed that UVB-induced DNA damage in the form of CPDs was removed or repaired faster in wild-type mice than IL-12p35-/- mice (Meeran et al., 2006c). In another experiment, IL-12p40-/- and their wild-type mice were exposed to UVB three times a week for four weeks, and thereafter mice were sacrificed, skin samples obtained and subjected to immunohistochemical analysis of CPDs. The staining of the nuclei for CPDs was more pronounced in skin of UV-exposed IL-12p40-/- mice than the skin of wild-type mice (Maeda et al., 2006). This further indicates that the amounts of UV-induced CPDs are higher in the absence of IL-12 presumably due to decreased DNA repair.
UVB-induced sunburn cell formation is primarily a consequence of DNA damage. Sunburn cells are keratinocytes undergoing apoptosis after they have received a physiological UV dose that irreversibly and severely damaged their DNA or other chromophores. IL-12 has been shown to have the ability to repair UVB-induced DNA damage and thus inhibits sunburn cell formation in the mouse epidermis (Schwarz et al., 2002; Meeran et al., 2006c). The inhibition of UV-induced apoptosis or sunburn cell formation by IL-12 may be due to a reduction in UV-induced DNA damage that, in turn, may lead to the reduction in photocarcinogenesis. Using IL-12 KO mice and their wild-type counterparts as a tool, Meeran et al. (2006c) determined the repair kinetics of UVB-induced sunburn cells in these mice. IL-12 KO mice and their wild-type were exposed to UVB radiation. Mice were sacrificed at different time points after UVB irradiation, skin samples were collected and processed for H & E staining for the detection of sunburn cells microscopically. It was observed that the numbers of sunburn cells in wild-type mice were significantly decreased at 24 hours and 48 hours after UV irradiation. However, the reduction in sunburn cells in IL-12 KO mice occurred slowly and was significantly less than that observed in wild-type mice at each time point studied. The higher numbers of sunburn cells in IL-12 KO mice does not indicate a reduced risk for photocarcinogenesis because the increase of apoptosis in this case may be related to enhanced amounts of DNA damage that may reflect lower levels of DNA repair. The difference in kinetics of the presence of sunburn cells between wild-type and IL-12 KO mice may be due to the endogenous presence and absence of IL-12, respectively, in wild-type and IL-12 KO mice. In an additional study, recombinant IL-12 was administered to IL-12 KO mice by subcutaneous injection at the experimental skin site three hours prior to UVB-irradiation. In this treatment group, the percentage of sunburn cells was significantly less than the percentage of sunburn cells in IL-12 KO mice that were not treated with recombinant IL-12 (Meeran et al., 2006c). The data from this experiment further provide evidence that the presence of IL-12 may have a role in removal or repair of UVB-induced DNA damage and this effect may result in lower risk of photocarcinogenesis in wild-type mice while enhancing the risk in IL-12 KO mice.
IL-12-deficiency enhances malignant transformation of UVB-induced papillomas to carcinomas
Malignant transformation of UVB-induced papillomas to carcinomas was recorded on a weekly basis in IL-12 KO mice and their wild-types. Compared with the wild-type mice, a significantly higher percentage of the IL-12 KO mice had carcinoma at the termination of the experiment at 35 weeks (Meeran et al., 2006c). The kinetics of malignant conversion of papillomas into carcinoma was also significantly higher in IL-12 KO mice than their wild-type counterparts. Thus, the risk of malignant progression of UVB-induced papilloma into carcinoma was higher in IL-12 KO mice than in their wild-type counterparts. The size of the individual carcinomas in IL-12 KO mice was larger than in wild-type mice. Interestingly, the carcinomas that developed in the IL-12 KO mice tended to occur in clusters of three to four whereas this clustering tendency was not apparent in the wild-type mice. It was intriguing to note that some of the skin tumors that occurred in IL-12 KO mice transformed rapidly into squamous cell carcinomas (Meeran et al., 2006c). These data clearly indicate that IL-12-deficient mice are not only extremely susceptible to UV-induced tumorigenesis, but they are extremely susceptible to progression of papillomas to carcinomas.
IL-12-deficiency enhances tumor cell proliferation
As it was observed that IL-12-deficient mice develops larger tumors after chronic UVB irradiation than wild-type mice, it is possible that IL-12-deficiency might enhance tumor cell proliferation in UV-induced skin tumors. To investigate this feature, tumor cells were isolated from skin tumors developed in IL-12 KO mice and their wild-type counterparts and subjected to analysis of their proliferative capacity. The proliferative capacity of the cultured tumor cells obtained from IL-12 KO mice was significantly higher than that of the cultured tumor cells obtained from the tumors of wild-type mice when analyzed using an MTT assay and colony formation assay (Maeda et al., 2006). In addition, the levels of expression of the proliferation-specific protein, cyclin D1, were higher in the UVB-induced tumors than in age-matched skin samples from unirradiated control mice of the same strain, and the levels of cyclin D1 were markedly higher in the UVB-induced tumors of IL-12 KO mice than the UVB-induced tumors of their wild-type counterparts (Katiyar et al., unpublished data). Additionally, the tumor cells obtained from the IL-12 KO mice secreted remarkably enhanced levels of pro-inflammatory cytokine, IL-6, in comparison with tumor cells obtained from wild-type mice. Higher production of this proinflammatory cytokine has been associated with increased tumor growth or metastasis in a variety of neoplasms (Smith et al., 1998). IL-6 has been reported to induce matrix metalloproteinases, which by themselves promote angiogenesis and invasion by acting on extracellular matrix proteins (Sundelin et al., 2005).
Effect of IL-12 in human cutaneous malignancies: outcome of clinical trials
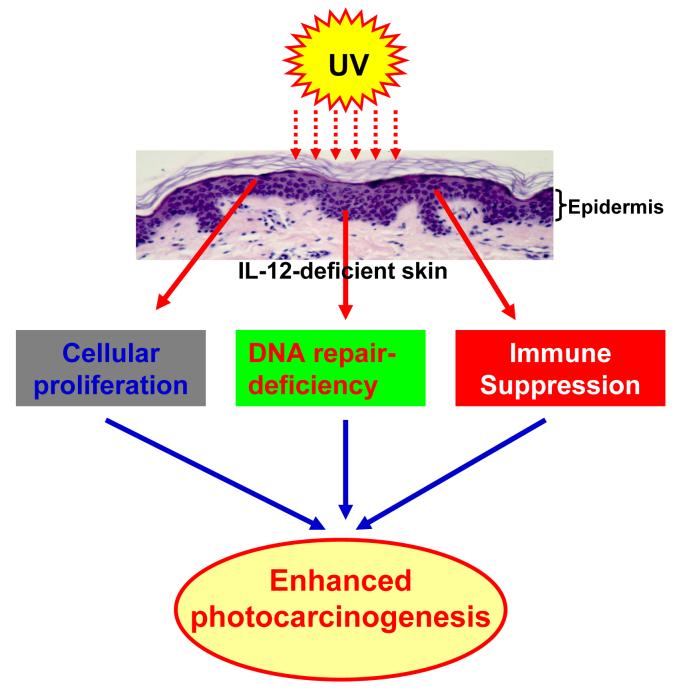
The present review of literature suggests that IL-12-deficiency enhances immune suppression, reduces UVB-induced damaged-DNA repair ability and enhances tumor cell proliferation in mice which leads to an enhanced risk of photocarcinogenesis (Fig. 2). To examine the potent chemotherapeutic effect of IL-12 against cutaneous malignancies in humans, some clinical trials have been conducted. In the human melanoma model, treatment with DNA coding for IL-12 induced regression of tumors in all cases, with complete disappearance of the tumor in two out of five animals. An antivascular effect of IL-12 treatment was evident on histological examination with endothelial thickening and abrupt changes in vessel diameters (Heinzerling et al., 2002). This study suggests that intratumoral plasmid DNA coding for IL-12 holds some promise as a new therapeutic tool for accessible melanoma lesions and should be tested in clinical trial. Rook et al. (1999) conducted a Phase I dose escalation trial with recombinant human IL-12 (rhIL-12) where patients suffering from cutaneous T-cell lymphoma were given rhIL-12 twice weekly subcutaneously or intralesionally for up to 24 weeks. It was observed that subcutaneous dosing resulted in complete responses in 2 of 5 plaque and partial responses in 2 of 5 plaque, and 1 of 2 Sezary syndrome patients. Intralesional dosing resulted in individual tumor regression in 2 of 2 patients. Adverse effects of rhIL-12 on this regimen were minor and limited and included low grade fever and headache. The results from this phase I trial suggest that rhIL-12 augments antitumor cytotoxic T-cell responses and may represent a potent and well-tolerated therapeutic agent for cutaneous T-cell lymphoma. Further phase I and phase II trials conducted by the same group with rhIL-12 against cytotoxic T-cell lymphoma revealed that an overall response rate approaching 50 percent. These results suggested that rhIL-12 induced lesion regression by augmenting antitumor cytotoxic T cell response (Rook et al., 2001). Another clinical trial was conducted in melanoma patients. In this study rhIL-12 was administered subcutaneously and intravenously. IL-12 therapy was well tolerated. Clinical responses included a complete response in a subject with small volume subcutaneous disease, a partial response in a subject with hepatic metastases. The efficacy of IL-12 was also tested in the patients with malignant melanoma. In this study, the plasmid DNA encoding human IL-12 was injected into lesions of nine patients. The therapy was well tolerated. Three of nine patients experienced a clinical response: two stable disease and one complete remission (Heinzerling et al., 2005). All patients but one experienced a transient response at the intratumoral injection site. These results show that intratumoral injection of DNA encoding IL-12 produced some beneficial clinical effect.
Fig. 2.
IL-12-deficiency enhances tumor cell proliferation, immune suppression and reduces UVB-induced damaged-DNA repair ability which leads to enhanced photocarcinogenesis in mice. Epidermis is the origin of melanoma and nonmelanoma skin cancers and keratinocytes are the major cell population (>90%) in it. Epidermal Langerhans cells and keratinocytes are capable to secrete or synthesize IL-12.
Conclusion and future prospects
The present information on IL-12 and photocarcinogenesis are consistent with the reports that IL-12 has the ability to cure or improve the survival of tumor-bearing mice and is associated with the enhancement of in vivo anti-tumor immune responses and anti-tumor activity in a number of other tumor models (Brunda et al., 1993; Nastala et al., 1994), with the presence of IL-12 at the tumor site being critical for tumor regression (Colombo et al., 1996). As endogenous production of IL-12 may inhibit UVB-induced skin carcinogenesis, it is tempting to speculate that the stimulation of immunoregulatory cytokine IL-12 in in vivo system by any mechanism, such as through dietary supplements, topical treatments, or IL-12 therapy, may prove efficacious in the prevention and treatment of solar UV radiation-induced skin cancers in humans. Some attempts are under way to use IL-12-based therapy of malignancies. Novel methods for IL-12 delivery include cell-based ex vivo gene therapy, viral vector-based gene therapy and DNA plasmid-based non-viral gene therapy. IL-12 electroporation gene therapy may hold some promise for tumors accessible by electrode, such as head and neck cancer, melanoma and nonmelanoma skin cancers. Co-delivery of other therapeutic genes with IL-12 may enhance the therapeutic effect and reduce the level of IL-12 required for efficacy.
Acknowledgments
The work reported from the author’s laboratory was supported from the funds from National Institutes of Health (CA104428, CA105368, CA089738, AT002536, ES11421), VA Merit Review Award, and UAB Skin Diseases Research Center (AR050948-01). The content of this publication does not necessarily reflect the views or policies of the funding sources. I feel honored to dedicate this review article to Prof. Hasan Mukhtar, University of Wisconsin, Madison, WI, on his 60th birthday.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: There are no conflicts of interest.
References
- Applegate LA, Ley RD, Alcalay J, Kripke ML. Identification of molecular targets for the suppression of contact hypersensitivity by ultraviolet radiation. J. Exp. Med. 1989;170:1117–1131. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin-12 against murine tumors. J. Exp. Med. 1993;178:1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RS, Cooper KD, De Fabo EC, Frederick JE, Gelatt KN, Hammond SP, Hersey P, Koren HS, Ley RD, Noonan F. Solar ultraviolet radiation and the risk of infectious disease. Photochem. Photobiol. 1995;61:223–247. doi: 10.1111/j.1751-1097.1995.tb03966.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Chen D, Bloack E, O’Donnell M, Kufe DW, Clinton SK. Eradication of murine bladder carcinoma by intratumor injection of a bicistronic adenoviral vector carrying cDNAs for the IL-12 heterodimer and its inhibition by the p40 subunit homodimer. J. Immunol. 1997;159:351–359. [PubMed] [Google Scholar]
- Colombo MP, Vagliani M, Spreafico F, Parenza M, Chiodoni C, Melani C, Stoppacciaro A. Amount of interleukin 12 available at the tumor site is critical for tumor regression. Cancer Res. 1996;56:2531–2534. [PubMed] [Google Scholar]
- Cowen EW, Billingsley EM. Awareness of skin cancer by kidney transplant patients. J. Am. Acad. Dermatol. 1999;40:697–701. doi: 10.1016/s0190-9622(99)70149-0. [DOI] [PubMed] [Google Scholar]
- DiGiovanna JJ. Posttransplantation skin cancer: scope of the problem, management and role for systemic retinoid chemoprevention. Transplant Proc. 1998;30:2771–2775. doi: 10.1016/s0041-1345(98)00806-9. [DOI] [PubMed] [Google Scholar]
- Donawho CK, Kripke ML. Evidence that the local effect of ultraviolet radiation on the growth of murine melanomas is immunologically mediated. Cancer Res. 1991;51:4176–4181. [PubMed] [Google Scholar]
- Fortina AB, Caforio AL, Piaserico S, Alaibac M, Tona F, Feltrin G, Livi U, Peserico A. Skin cancer in heart transplant recipients: frequency and risk factor analysis. J. Heart Lung Transplant. 2000;19:249–255. doi: 10.1016/s1053-2498(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Halak BK, Maguire HC, Jr., Lattime EC. Tumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a non-tumor antigen present at the tumor site. Cancer Res. 1999;59:911–917. [PubMed] [Google Scholar]
- Hayes MP, Wang J, Norcross MA. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- Heinzerling L, Burg G, Dummer R, Maier T, Oberholzer PA, Schultz J, Elzaouk L, Pavlovic J, Moelling K. Intratumoral injection of DNA encoding human interleukin 12 into patients with metastatic melanoma: clinical efficacy. Hum. Gene Ther. 2005;16:35–48. doi: 10.1089/hum.2005.16.35. [DOI] [PubMed] [Google Scholar]
- Heinzerling L, Dummer R, Pavlovic J, Schultz J, Burg G, Moelling K. Tumor regression of human and murine melanoma after intratumoral injection of IL-12-encoding plasmid DNA in mice. Exp. Dermatol. 2002;11:232–240. doi: 10.1034/j.1600-0625.2001.110306.x. [DOI] [PubMed] [Google Scholar]
- Katiyar SK. Oxidative stress and photocarcinogenesis: Strategies for prevention. In: Singh KK, editor. Oxidative Stress, Disease and Cancer. Imperial College Press; London: 2006. pp. 933–964. [Google Scholar]
- Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J. Natl. Cancer Inst. 1997;89:556–566. doi: 10.1093/jnci/89.8.556. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Mukhtar H. Green tea polyphenol (-)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen presenting cells and oxidative stress. J. Leukoc. Biol. 2001;69:719–726. [PubMed] [Google Scholar]
- Kinlen L, Sheil A, Peta J, Doll R. Collaborative United Kingdom-Australia study of cancer in patients treated with immunosuppressive drugs. Br. J. Med. 1979;II:1461–1466. doi: 10.1136/bmj.2.6203.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke ML. Photoimmunology. Photochem. Photobiol. 1990;52:919–923. doi: 10.1111/j.1751-1097.1990.tb08703.x. [DOI] [PubMed] [Google Scholar]
- Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiated systemic immunosuppression in UV-irradiated mice. Proc. Natl. Acad. Sci. USA. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Schneider SW, Kojima M, Beissert S, Schwarz T, Schwarz A. Enhanced photocarcinogenesis in interleukin-12-deficient mice. Cancer Res. 2006;66:2962–2969. doi: 10.1158/0008-5472.CAN-05-3614. [DOI] [PubMed] [Google Scholar]
- Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J. Exp. Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner F, Fischer S, Guckes S, Jin S, Kaulen H, Schmitt E, Rude E, Germann T. The interleukin-12 subunit p40 specifically inhibits effects of the interleukin-12 heterodimer. Eur. J. Immunol. 1993;23:2202–2208. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- Meeran SM, Katiyar S, Elmets CA, Katiyar SK. Silymarin inhibits UV radiation-induced immunosuppression through augmentation of interleukin-12 in mice. Mol. Cancer Ther. 2006;5:1660–1668. doi: 10.1158/1535-7163.MCT-06-0095. [DOI] [PubMed] [Google Scholar]
- Meeran SM, Mantena SK, Katiyar SK. Prevention of ultraviolet radiation-induced immunosuppression by (-)-epigallocatechin-3-gallate in mice is mediated through interleukin 12-dependent DNA repair. Clinical Cancer Res. 2006a;12:2272–2280. doi: 10.1158/1078-0432.CCR-05-2672. [DOI] [PubMed] [Google Scholar]
- Meeran SM, Mantena SK, Elmets CA, Katiyar SK. (-)-Epigallocatechin-3-gallate prevents photocarcinogenesis in mice through interleukin-12-dependent DNA repair. Cancer Res. 2006b;66:5512–5520. doi: 10.1158/0008-5472.CAN-06-0218. [DOI] [PubMed] [Google Scholar]
- Meeran SM, Mantena SK, Meleth S, Elmets CA, Katiyar SK. Interleukin-12-deficient mice are at greater risk of ultraviolet Radiation-induced skin tumors and malignant transformation of papillomas to carcinomas. Mol. Cancer Ther. 2006c;5:825–832. doi: 10.1158/1535-7163.MCT-06-0003. [DOI] [PubMed] [Google Scholar]
- Meunier L, Raison-Peyron N, Meynadier J. UV-induced immunosuppression and skin cancers. Rev. Med. Interne. 1998;19:247–254. doi: 10.1016/S0248-8663(97)89326-5. [DOI] [PubMed] [Google Scholar]
- Mu J, Zou JP, Yamamoto N, Tsutsui T, Tai XG, Kobayashi M, Herrmann S, Fujiwara H, Hamaoka T. Administration of recombinant interleukin 12 prevents outgrowth of tumor cells metastasizing spontaneously to lung and lymph nodes. Cancer Res. 1995;55:4404–4408. [PubMed] [Google Scholar]
- Mukhtar H, Elmets CA. Photocarcinogenesis: mechanisms, models and human health implications. Photochem. Photobiol. 1996;63:355–447. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Muller G, Saloga J, Germann T, Schuler G, Knop J, Enk AH. IL-12 as mediator and adjuvant for the induction of contact sensitivity in vivo. J. Immunol. 1995;155:4661–4668. [PubMed] [Google Scholar]
- Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, Gately MK, Wolf SF, Schreiber RD, Storkus WJ. Recombinant IL-12 administration induces tumor-regression in association with IFN-γ production. J. Immunol. 1994;153:1697–1706. [PubMed] [Google Scholar]
- Otley CC, Pittelkow MR. Skin cancer in liver transplant recipients. Liver Transpl. 2000;6:253–262. doi: 10.1053/lv.2000.6352. [DOI] [PubMed] [Google Scholar]
- Robertson MJ, Ritz J. Interleukin-12: basic biology and potential applications in cancer treatment. Oncologist. 1996;1:88–97. [PubMed] [Google Scholar]
- Romerdahl CA, Okamoto H, Kripke ML. Immune surveillance against cutaneous malignancies in experimental animals. Immunol. Ser. 1989;46:749–767. [PubMed] [Google Scholar]
- Rook AH, Wood GS, Yoo EK, Elenitsas R, Kao DM, Sherman ML, Witmer WK, Rockwell KA, Shane RB, Lessin SR, Vonderheid EC. Interleukin-12 therapy of cutaneous T-cell lymphoma induces lesion regression and cytotoxic T-cell responses. Blood. 1999;94:902–908. [PubMed] [Google Scholar]
- Rook AH, Zaki MH, Wysocka M, Wood GS, Duvic M, Showe LC, Foss F, Shapiro M, Kuzel TM, Olsen EA, Vonderheid EC, Laliberte R, Sherman ML. The role for interleukin-12 therapy of cutaneous T cell lymphoma. Ann. N Y Acad. Sci. 2001;941:177–184. doi: 10.1111/j.1749-6632.2001.tb03721.x. [DOI] [PubMed] [Google Scholar]
- Schmitt DA, Owen-Schaub L, Ullrich SE. Effect of IL-12 on immune suppression and suppressor cell induction by ultraviolet radiation. J. Immunol. 1995;154:5114–5120. [PubMed] [Google Scholar]
- Schwarz T. Mechanism of UV-induced immunosuppression. Keio J. Med. 2005;54:165–171. doi: 10.2302/kjm.54.165. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Grabbe S, Aragane Y, Sandkuhl K, Riemann H, Luger TA, Kubin M, Trinchieri G, Schwarz T. Interleukin-12 prevents ultraviolet B-induced local immunosuppression and overcomes UVB-induced tolerance. J. Invest. Dermatol. 1996;106:1187–1191. doi: 10.1111/1523-1747.ep12347944. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Maeda A, Kernebeck K, van Steeg H, Beissert S, Schwarz T. Prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. J. Exp. Med. 2005;201:173–179. doi: 10.1084/jem.20041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz A, Stander S, Berneburg M, Bohm M, Kulms D, van Steeg H, Grosse-Heitmeyer K, Krutmann J, Schwarz T. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nat. Cell Biol. 2002;4:26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- Sharma SD, Katiyar SK. Dietary grape-seed proanthocyanidin inhibition of ultraviolet B-induced immune suppression is associated with induction of IL-12. Carcinogenesis. 2006;27:95–102. doi: 10.1093/carcin/bgi169. [DOI] [PubMed] [Google Scholar]
- Siders W, Wright P, Hixon J, Alvord WG, Back TC, Wiltrout RH, Fenton RG. T cell- and NK cell-independent inhibition of hepatic metastases by systemic administration of an IL-12-expressing recombinant adenovirus. J. Immunol. 1998;160:5465–5474. [PubMed] [Google Scholar]
- Smith CW, Chen Z, Dong G, Loukinova E, Pegram MY, Nicholas-Figueroa L, Van Waes C, Smith CW, Chen Z, Dong G, Loukinova E, Pegram MY, Nicholas-igueroa L, Van Waes C. The host environment promotes the development of primary and metastatic squamous cell carcinomas that constitutively express proinflammatory cytokines IL-1alpha, IL-6, GM-CSF, and KC. Clin. Exp. Metastasis. 1998;16:655–664. doi: 10.1023/a:1006559811429. [DOI] [PubMed] [Google Scholar]
- Sundelin K, Roberg K, Grenman R, Hakansson L. Effects of cytokines on matrix metalloproteinase expression in oral squamous cell carcinoma in vitro. Acta Otolaryngol. 2005;125:765–773. doi: 10.1080/00016480510027484. [DOI] [PubMed] [Google Scholar]
- Tahara H, Zeh HJ, 3rd, Storkus WJ, Pappo I, Watkins SC, Gubler U, Wolf F, Robbins PD, Lotze MT. Fibroblasts genetically engineered to secrete interleukin 12 can suppress tumor growth and induce antitumor immunity to a murine melanoma in vivo. Cancer Res. 1994;54:182–189. [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- Trinchieri G, Scott P. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions. Res. Immunol. 1995;146:423–431. doi: 10.1016/0923-2494(96)83011-2. [DOI] [PubMed] [Google Scholar]
- Ullrich SE. Potential for immunotoxicity due to environmental exposure to ultraviolet radiation. Hum. Exp. Toxicol. 1995;14:89–91. doi: 10.1177/096032719501400118. [DOI] [PubMed] [Google Scholar]
- Urbach F. Incidences of nonmelanoma skin cancer. Dermatol. Clin. 1991;9:751–755. [PubMed] [Google Scholar]
- Van der Leun JC. In: Human Health: United Nations Environmental Program report on the Environmental Effects of ozone Depletion. Van der Leun JC, Tevini M, editors. EPA; Washington, DC: 1989. [Google Scholar]
- Vink AA, Moodycliffe AM, Shreedhar V, Ullrich SE, Roza L, Yarosh DB, Kripke ML. The inhibition of antigen-presenting activity of dendritic cells resulting from UV irradiation of murine skin is restored by in vitro photorepair of cyclobutane pyrimidine dimers. Proc. Natl. Acad. Sci. U S A. 1997;94:5255–5260. doi: 10.1073/pnas.94.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink AA, Strickland FM, Bucana C, Cox PA, Roza L, Yarosh DB, Kripke ML. Localization of DNA damage and its role in altered antigen-presenting cell function in ultraviolet-irradiated mice. J. Exp. Med. 1996;183:1491–1500. doi: 10.1084/jem.183.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voest EE, Kenyon BM, O’Reilly MS, Truitt G, D’Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J. Natl. Cancer Inst. 1995;87:581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- Wigginton JM, Komschlies KL, Back TC, Franco JL, Brunda MJ, Wiltrout RH. Administration of interleukin 12 with pulse interleukin 2 and the rapid and complete eradication of murine renal carcinoma. J. Natl. Cancer Inst. 1996;88:38–43. doi: 10.1093/jnci/88.1.38. [DOI] [PubMed] [Google Scholar]
- Wolf SF, Temple PA, Kobayashi M, Young D, Dicig M, Lowe L, Dzialo R, Fitz L, Ferenz C, Hewick RM. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J. Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- Yarosh D, Alas LG, Yee V, Oberyszyn A, Kibitel JT, Mitchell D, Rosenstein R, Spinowitz A, Citron M. Pyrimidine dimer removal enhanced by DNA repair liposomes reduces the incidence of UV skin cancer in mice. Cancer Res. 1992;52:4227–4231. [PubMed] [Google Scholar]
- Yoshikawa T, Rae V, Bruins-Slot W, vand-den-Berg JW, Taylor JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J. Invest. Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacks T, Brash DE. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- Zou JP, Yamatato N, Fuzii T, Takenaka H, Kobayashi M, Herrmann SH, Wolf SF, Fujiwara H, Hamaoka T. Systemic administration of rIL-12 induces complete tumor regression and protective immunity: response is correlated with a striking reversal of suppressed IFN-γ production by anti-tumor T cells. Int. Immunol. 1995;7:1135–1145. doi: 10.1093/intimm/7.7.1135. [DOI] [PubMed] [Google Scholar]