Introduction
PITPs transfer PtdIns or PtdCho monomers between membrane bilayers in vitro (Cleves et al 1991a; Kearns et al., 1998a; Wirtz, 1991). While a convenient biochemical assay for protein ‘activity’, it remains unclear how PtIns- or PtdCho-transfer activity is translated into biological function. This is an important set of issues as insufficiencies in the activities of individual PITPs lead anywhere from in viability of single yeast cells, to tissue-specific developmental defects in plants, to neurodegenerative and malabsorbtion disease in mammals. The known PITPs fall into two groups, the Sec14-like PITPs and the metazoan PITPs. The latter are so termed because, with the exception of dictyostelium, these are found exclusively in metazoan organisms (Table 1). Whereas even the complicated mammalian cells express only a few metazoan PITPs, the simplest eukaryotic cells express multiple Sec14-like proteins and the scale of expression of these proteins increases in a manner commensurate with the complexity of the organism. The Sec14-like proteins form a eukaryotic protein superfamily of greater than 500 members presently known. Yet, this superfamily in general remains rather poorly studied.
Table 1.
| Sec14 protiens | Metazoan PITPs |
|
|---|---|---|
| S.cerevisiae | 6 | 0 |
| C.elegans | >15 | 3 |
| D.melanogaster | >15 | 3 |
| M.musculus | >23 | 5 |
| H.sapiens | >23 | 5 |
| A.thaliana | 31 | 0 |
| D.discoideum | 4 | 4 |
Structural studies provide some of the first details regarding the architecture of Sec14-like proteins (Sha et al., 1998; Phillips et al., 1999; Stocker et al., 2002; Min et al., 2003). The Sec14-fold is a unique one and, while structures for ligand-bound Sec14-like proteins have been solved, none of these are of phospholipid-binding members of the superfamily. Thus, how Sec14-like proteins, particularly Sec14-like PITPs, bind phospholipids is unknown. While recent electron spin resonance studies outline some general rules for how Sec14p binds its phospholipid substrates (Smirnova et al., 2006), high resolution crystal structures of phospholipid-bound Sec14p (or Sec14-like proteins) are required. The available Sec14p structures describe what likely resembles a transitional Sec14p conformation found on membrane surfaces upon release of bound phospholipid and prior to reloading with another phospholipid molecule. The basic details for how the structural elements of Sec14-like PITPs endow these proteins with the ability to recognize their specific binding substrates, and what conformational transitions are needed for the efficient abstraction of the cognate phospholipid ligands from a membrane, remain to be determined. These are important questions because of the curious biochemistry of the transfer reaction itself. Sec14p, and other PITPs, are able to extract a phospholipid molecule from a membrane bilayer in an ATP- and cofactor-independent manner. The biochemistry of PITP-mediated phospholipid transfer is even more remarkable considering this reloading reaction occurs after Sec14p has ejected a bound phospholipid from its hydrophobic binding pocket.
From the standpoint of specificity of biological function, the yeast Saccharomyces cerevisae expresses six Sec14-like proteins. These include Sec14p itself, the founding member of the Sec14 protein superfamily, and five other Sec Fourteen Homologs (SFH proteins). The flowering mustard weed Arabidopsis expresses thirty-one Sec14-like proteins while metazoans express in excess of twenty each (Table 1). Why so many Sec14-like proteins? The emerging concept is that Sec14-like PITPs constitute important components of specific signaling nano-machines, or nanoreactors (see Ile et al., 2006). This concept has significant implications for how enzyme activity is regulated in eukaryotic cells. Herein, we discuss the evidence to support such a role for Sec14-like PITPs in helping shape the landscape of lipid signaling in eukaryotic cells.
Materials and methods
The experimental procedures and reagents employed herein are described in (Rivas et al., 1999; Li et al., 2000; Wu et al., 2000; Xie et al., 2001; Routt et al., 2005; Vincent et al., 2005).
Results and discussion
The budding yeast Sec14p
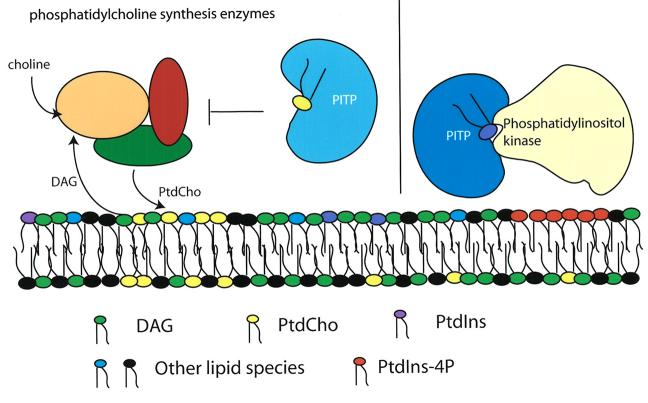
A substantial body of functional data demonstrates that the essential role of Sec14p in regulating protein transport from the yeast trans-Golgi network is to coordinate the interface between lipid metabolism and the action of core protein components of the vesicle budding machinery (Bankaitis et al., 1990; Cleves et al., 1991a, 1991b; Kearns et al., 1997; Huijbregts et al., 2000; Henneberry et al., 2001; Ile et al., 2006; Phillips et al., 2006). The present model that describes the basic elements of this regulatory circuit is shown in Figure 1. Briefly, we posit that the PtdCho-bound form of Sec14p functions to reduce the metabolic flux through the CDP-choline pathway for PtdCho biosynthesis. This aspect of the model depicted in Figure 1 is motivated by the unanticipated discovery that individual inactivation of the structural genes encoding enzymes of this biosynthetic pathway have the effect of liberating cells from the essential requirement of Sec14p for membrane trafficking from the TGN and cell viability (Cleves et al., 1991b; McGee et al., 1994; Skinner et al., 1995). The specificity of the functional relationship between Sec14p and activity of the CDP-choline pathway is remarkable. Inactivation of the parallel phosphatidylethanolamine methylation pathway for PtdCho biosynthesis exerts no such effect except under special growth conditions that themselves prevent active metabolic flux through the CDP-choline pathway (Xie et al., 2001). Analyses of headgroup-specific phospholipid binding mutants of Sec14p indicate this regulation of PtdCho biosynthesis is a particularly important function for Sec14p (Phillips et al., 1999). We also posit the PtdIns-bound Sec14p functions independently, and that the two functions converge in the TGN vesicle biogenetic pathway. Specifically, Sec14p stimulates PtdIns 4-OH kinase activity in vivo, and genetic inactivation of either the Sac1p phosphoinositide phosphatase (Cleves et al., 1989, 1991a; Whitters et al., 1993; Guo et al., 1999; Hama et al., 1999; Rivas et al., 1999) or a sterol- and PtdIns-4-phosphate binding protein (Kes1p) also frees yeast cells from the essential Sec14p requirement for TGN secretory function and cell viability (Fang et al., 1996; Li et al., 2002; Im et al., 2005).
Fig. 1.
Sec14p regulates both PtdCho and phosphoinositide biosynthesis. Sec14p coordinates the interface between lipid metabolism and transport vesicle biogenesis from yeast trans-Golgi membranes. Sec14p-mediated regulation of lipid metabolism is twofold: inhibition of PtdCho biosynthesis and diacylglycerol (DAG) consumption via the CDP-choline pathway (left panel) and stimulation of PtdIns-4-phosphate production via the Pik1p PtdIns 4-OH kinase.
What proteins are the targets of this lipid-mediated regulation? The available evidence points to the ARF small GTPase cycle, in particular a specific pair ARFGAPs (Gcs1p and Age2p) whose membrane recruitment and lipid-regulated activation sets an ARF-dependent positive feedback loop that loads membranes with ARFGAP at the site where a vesicle will form (Li et al., 2002; Yanagisawa et al., 2002; Phillips et al., 2006). We propose Sec14p bound to PtdCho is of primary importance at this specific execution point. The ability of ARFGAPs to physically interact with core proteins of the vesicle budding machinery (see Yang et al., 2002; Lewis et al., 2004) set in motion the subsequent propagation of protein-protein interactions that ultimately generate a functional vesicle (Phillips et al., 2006; Ile et al., 2006). We propose Sec14p bound to PtdIns is of primary importance at this later execution point. A thorough test of the model presented here demands the generation of mutant Sec14ps specifically defective in PtdCho-binding/transfer. The prediction is these types of mutant Sec14ps will not score as fully functional proteins.
Functionally distinct Sec14p-like proteins in Saccharomyces cerevisiae
As indicated above, the simple budding yeast expresses Sec14p and five other Sec14-like proteins designated Sfh1p - Sfh5p. Sfh1p shares 64% primary sequence identity with Sec14p but, surprisingly, does not exhibit significant functional redundancy or biochemical similarity with Sec14p. This is made even more puzzling given that the remaining four SFH proteins are ca. 25% identical and 45% similar to Sec14p in primary sequence. Yet, specific phospholipid transfer activities can be assigned to Sfh2p, Sfh4p and Sfh5p. These phospholipid transfer activities identify these SFH proteins as non-classical PITPs in that these proteins transfer PtdIns but not PtdCho in vitro (Li et al., 2000). With regard to in vivo function, the SFH proteins are unlike Sec14p in that these are neither individually nor collectively essential for cell viability. Rather, the SFH proteins stimulate phospholipase D (PLD) activity in vegetative yeast cells and also the activities of phopholipases B (Li et al., 2000; Schnabl et al., 2003). The functional relationship between SFH proteins and PLD, considering the ability of SFH proteins to transfer PtdIns, suggests that SFH proteins exert their function by stimulating PtdIns(4,5)P2 production. Indeed, the SFH proteins do stimulate synthesis 4-OH phosphoinositides in vivo, and this effect is mediated through a particular PtdIns 4-OH kinase (i.e. the STT4 gene product; Routt et al., 2005).
Although individual overexpression of Sfh2p, Sfh4p and to a lesser extent Sfh5p rescues growth and secretory defects in Sec14p insufficient yeast (Li et al., 2000; Schnabl et al., 2003), it is clear the SFH proteins are not functionally redundant with Sec14p in vivo. As reviewed below, these proteins have unique functions in their own right. This specification of function is intriguing from the standpoint that these functionally distinct SFH proteins all act through the same Stt4p PtdIns 4-OH kinase in vegetative cells, a property these share with Sec14p (Nemoto et al., 2001; Routt et al., 2005).
Sfh2p and oxidative stress
Recent work has identified a most interesting involvement of Sfh2p with oxidative stress responses in yeast. Cha et al. (2003) find that Sfh2p binds the yeast cytosolic thioperoxidase II (cTPII). This physical interaction is a specific one on multiple counts. First, Sfh2p interacts exclusively with the dimerized form of cTPII. The cTPII dimer represents the oxidized form of the enzyme and is therefore a detector for oxidative stress. Second, Sfh2p fails to bind any of the other three yeast thioperoxidase isoforms. Third, Sfh2p is the only one of the five yeast Sec14-like proteins capable of binding cTPII, and Sec14p does not bind this enzyme either. Fourth, double mutant yeasts defective in both cTPII and Sfh2p are more susceptible to oxidative stress. It would be interesting to determine whether the phospholipid-bound state of Sfh2p differentially affects cTPII activity, although recombinant Sfh2p fails to do so (Cha et al., 2003). Thus, the Sfh2p nanoreactor consists of Sfh2p and the cTPII dimer. What other activities may be involved remains an open question. It does raise the interesting possibility that the cTPII dimer regulates Sfh2p activity (rather than the converse) and that the Sfh2p nanoreactor couples to PtdIns kinases. In that regard, at least one soybean SFH protein is acutely phosphorylated by osmotic stress and is proposed to regulate osmotic stress responses (Kearns et al., 1998b). The role of Sec14-like proteins in stress responses is a promising arena for future research.
Sfh4p and intermembrane contact sites
All eukaryotic cells examined to date express a mitochondrial phosphatidylserine (PtSer) decarboxylase that converts PtdSer to phosphatidylethanolamine (PtdEtn), and a large body of biochemical evidence suggests the PtdSer substrate is mobilized from the endoplasmic reticulum (ER) to the mitochondrion, the trans-Golgi network, or the plasma membrane via intermembrane contact sites (Shiao et al., 1995; Achleitner et al., 1999; Marsh et al., 2001; Pichler et al., 2001). Study of such putative intermembrane contact sites, and contact sites in general, is hampered by their intractability to biochemical and imaging approaches. Recently, Dennis Voelker and colleagues devised a clever genetic screen for components of such contact sites in yeast (Trotter et al., 1998; Wu et al., 2000). One such component is Sfh4p (also described by the nonconventional designation PstB2p) on the basis of its identification in a genetic screen for mutants defective in the activity of a non-mitochondrial pathway for PtdSer decarboxylation (Wu et al., 2000). The available data suggest this Sfh4p-dependent pathway involves PtSer mobilization from the yeast ER to an endosomal PtdSer decarboxylase Psd2p (Wu and Voelker, 2002). As Sfh4p has no detectable PtdSer transfer activity, the present interpretation of the collective data is that Sfh4p ferries PtdSer monomers to Psd2p (Wu et al., 2000). PtdSer transport to Psd2p requires high concentrations of PtdSer in donor membranes, and the transfer reaction operates much more efficiently when the donor membrane is not highly curved (vesicle diameter ≥ 400nm; Wu and Voelker, 2004). These features suggest ER microdomains rich in PtdSer are the donor units for the transfer reaction. As Sfh4p is a membrane-associated protein (Li et al., 2000; Wu et al., 2000), its role in the process is most easily explained by an intermembrane contact site mechanism. Determination of the lifetimes (i.e. dynamics) of these sites, and the mechanisms for how these gate specific phospholipids, remains unclear.
While large questions remain outstanding regarding how Sfh4p executes biological function, what is abundantly clear is that it is unique amongst the budding yeast Sec14-like proteins in executing its function in the non-mitochondrial PtdSer decarboxylation pathway. Neither Sec14p, nor any other SFH protein, can fulfill the same role – even under conditions of substantial overexpression (Routt et al., 2005). Given that the Stt4p PtdIns 4-OH kinase is also identified in the same genetic screen that yielded Sfh4p (Trotter et al., 1998), and Sfh4p stimulated PtdIns-4-phosphate production in what appears to be an exclusively Stt4p-dependent manner (Routt et al., 2005), the simplest interpretation of the data is that Sfh4p cooperates with Stt4p in generating a phosphoinositide pool that is then somehow gainfully employed in extra-mitochondrial PtdSer decarboxylation. Because Sec14p and other SFH proteins also couple to Stt4p, the data further suggest a very specific and privileged functional interface of Sfh4p, Stt4p and other proteins (e.g. Psd2p) in a metabolic nanoreactor that converts PtdSer to PtdEtn (see Ile et al., 2006).
Sfh5p and plasma membrane PtdIns(4,5)P2 homeostasis
An involvement in the generation or maintenance of specific intermembrane contact sites may not be restricted to Sfh4p. Alternatively, there may exist distinct families of contact sites and their identities may be in part determined by the SFH proteins that populate (or otherwise regulate) them. There is some evidence consistent with the possibility that Sfh5p may do the same but, in this case, the contact site may bridge the peripheral ER and plasma membrane (Routt et al., 2005). What might the physiological function of such a contact site be? A combination of genetic and biochemical data suggests a functional Sfh5p-Stt4p interaction regulates the constitutive fusion of Golgi-derived secretory vesicles to the plasma membrane. Elevated gene dosage for SFH5, MSS4 (encodes the single yeast PtdIns-4-phosphate 5-OH kinase) or SEC9 (encodes a yeast t-SNARE) gene dosage levies significant rescue of certain late sec mutant phenotypes. Those data raise the tantalizing possibility that an Sfh5p/Stt4p/Mss4p pathway regulates a PtdIns(4,5)P2-mediated activation of t-SNARE activity via the Sec9p subunit of the plasma membrane t-SNARE complex. Since the available data indicates Sfh5p activity primarily manifests itself at the level of PtdIns(4,5)P2 (Routt et al., 2005), activation of the plasma membrane t-SNARE complex may involve a higher level organization that optimizes plasma membrane t-SNARE complexes into fusion-competent structures.
What makes the genetic interaction data intriguing is the subcellular localization of Sfh5p, Stt4p and Mss4p. Both Stt4p and Mss4p are localized to plasma membrane subdomains, but these subdomains are not coincident (Audhya and Emr, 2002). By contrast, Sfh5p localizes to peripheral ER and to subdomains of the peripheral ER that appear closely apposed to the plasma membrane (Routt et al., 2005). This arrangement of Sfh5p with respect to its partner PtdIns- and phosphoinositide kinases is consistent with a contact-site model for Sfh5p function. In terms of the nanoreactor concept, the contact site would minimally involve the Stt4p and Mss4p PtdIns kinases and the Sfh5p PITP subunit. Other proteins may be required for actual formation of the proposed contact-site and these too would be defined as components of the Sfh5p nanoreactor.
Multidomain Sec14-like proteins; the Sec14-nodulin proteins
Higher eukaryotes express a large number of interesting modular proteins with Sec14-domains. Of particular interest to this discussion are Sec14-nodulin and Sec14-“Golgi-dynamics” (GOLD) proteins. The latter are conserved throughout the multicellular eukaryotes and very little information exists regarding in vivo functions for multidomain Sec14-like proteins in general. This lack of information is largely the result of multi-domain Sec14 protein expression being limited to higher eukaryotes. The nodulin-domains under discussion here are predicted to represent coiled-coil modules, and these domains were first identified as free-standing proteins of ca. 16 kDa in molecular mass that are expressed exclusively in N2-fixing root nodules in leguminous plants (Kapranov et al., 1997). The non-leguminous flowering plant Arabidopsis thaliana harbors 31 genes (AtSFH genes) that encode Sec14p-like proteins (Sec Fourteen Homologs). Thirteen of these encode gene products with C-terminal nodulin domains and these 13 fall into the top 14 Arabidopsis proteins with the highest homology to yeast Sec14p (Figure 2; Vincent et al., 2005). Functional analyses of the Sec14-domains from Sec14-nodulin proteins demonstrate that all exhibit intrinsic Sec14p-like activity, and at least half of these exhibit both PtdIns- and PtdCho-transfer activity and the ability to stimulate phosphoinositide synthesis in a heterologous yeast model.
Fig. 2.
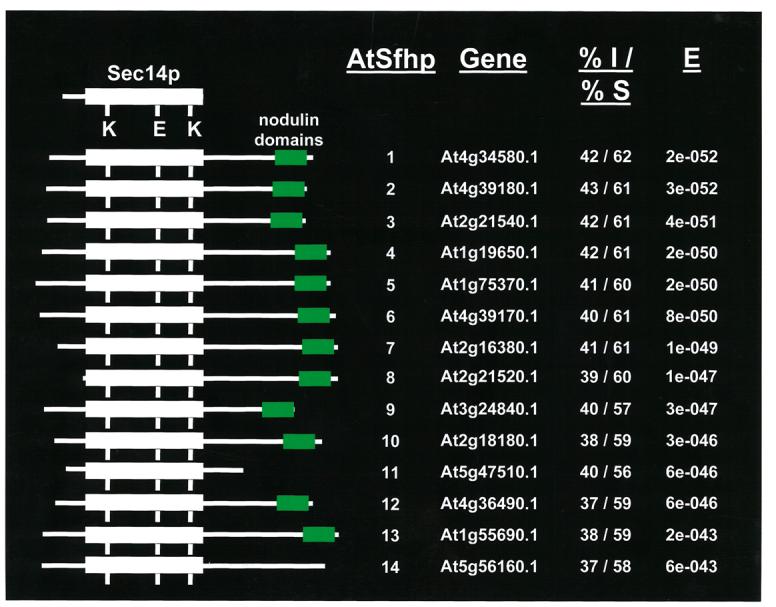
The Sec14-nodulin protein family in Arabidopsis. A schematic alignment of the Arabidopsis two-domain Sec14-nodulin proteins is shown. The Arabidopsis Information Resource database accession numbers for each are given, as are the corresponding Sec14-domain primary sequence identities/similarities to the yeast Sec14p. The corresponding e-values are also given.
The available evidence suggests that AtSFH proteins represent a novel class of regulators of polarized membrane trafficking. The evidence to this effect derives largely from the study of one of the Arabidopsis AtSFH proteins (AtSfh1p) and of its homolog in the legume Lotus japonicus (Kapranov et al., 2001, Vincent et al., 2005). Nullizygous (Atsfh1−/−) lines of Arabidopsis are fertile but, consistent with the root-specific expression profile of the AtSFH1 gene, elaborate abnormally short and stubby root hairs that often exhibit multiple growing tips (Vincent et al., 2005). An environmental scanning electron micrograph comparing root hair morphologies of wild-type vs Atsfh1−/− seedlings (3-day old) is shown in Figure 3. The Atsfh1−/− root hair phenotype is fully penetrant and manifests itself shortly after germination.
Fig. 3.
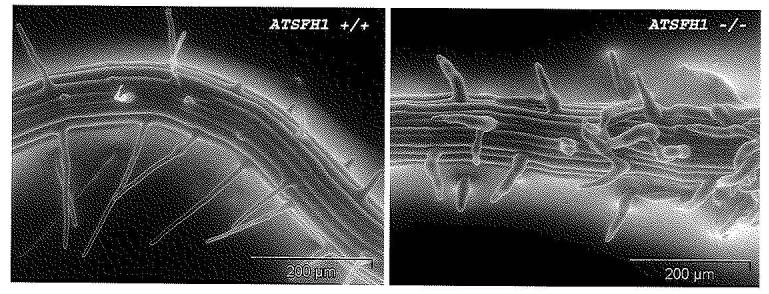
AtSfh1p deficiency results in defective root hair elongation. Environmental scanning electron microscopic imaging of living root hairs of 3 day-old wild-type (AtSFH1+/+; left panel) and AtSfh1p-deficient (AtSFH1−/−; right panel) Arabidopsis plants.
Sophisticated 3-dimensional image reconstruction studies indicate AtSfh1p controls specific pools of PtdIns-4-phosphate and PtdIns(4,5)P2 phosphoinositides in Arabidopsis, and that the distributions of both AtSfh1p and PtdIns(4,5)P2 in developing root hairs are most concentrated at the site of polarized membrane growth (Vincent et al., 2005). That is, both the GFP-AtSfh1p and phospholipase Cδ1 PH-domain-GFP reporters localize in similar patterns – i.e. heavy concentration in tip cytoplasm and a spiraling distribution on the root hair cortical plasma membrane (Vincent et al., 2005). These data raise the exciting possibility that regions of AtSfh1p localization may significantly overlap with areas of PtdIns(4,5)P2 enrichment, and that AtSfh1p promotes PtdIns(4,5)P2 synthesis and organization in root hair membrane ‘micro-domains’. This concept is supported by the demonstration that loss of AtSfh1p function results in loss of the PtdIns(4,5)P2 arrangement typical of growing wild-type root hairs (Vincent et al., 2005). How AtSfh1p promotes PtdIns-4-phosphate and PtdIns(4,5)P2 synthesis is unclear. It could occur via a direct stimulation of PtdIns-kinase activity, or through the activation of root hair phospholipase D isoforms (e.g. PLDζ) that indirectly activate PtdIns-4-phosphate 5-OH kinases via production of phosphatidic acid (Figure 4).
Fig. 4.
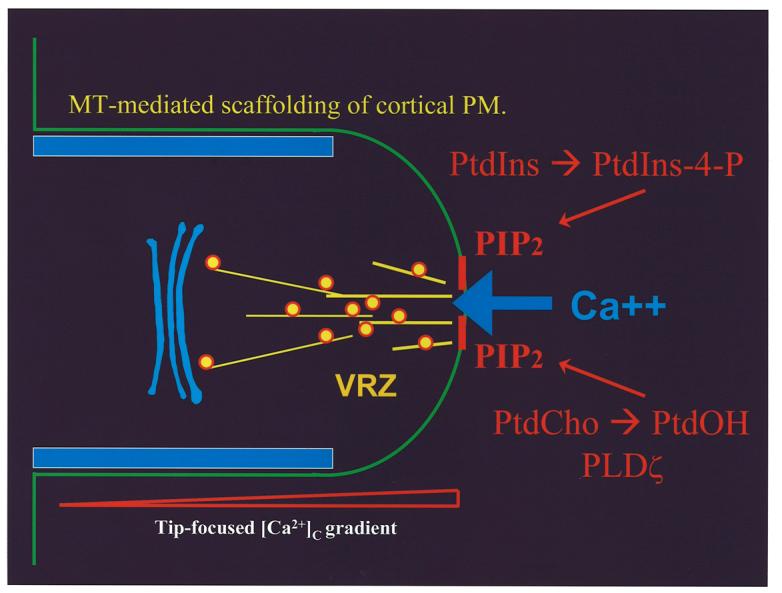
AtSfh1p-mediated regulation of polarized membrane growth. Our present model for how AtSfh1p helps generate and organize PtdIns(4,5)P2 domains on nascent secretory vesicles and the root tip plasma membrane. These domains regulate tip actin assembly that impose polarized vesicle trafficking and secretory cargo deposition to the tip plasma membrane. This, in turn, generates a tip-directed Ca++-gradient, and regulates microtubule assembly.
How might PtdIns(4,5)P2 synthesis and organization be gainfully employed in root hair biogenesis? The root hair cytoskeleton is a primary recipient of these regulatory cues given that the actin and microtubule cytoskeletal systems respond to this PtdIns(4,5)P2 organization. Derangement of PtdIns(4,5)P2 in Atsfh1−/− root hairs is accompanied by disruption of the tip actin microfilament network, but not of the cortical actin cytoskeleton. Since many actin binding proteins bind PtdIns(4,5)P2 with higher affinity than that with which these bind actin, it is perhaps not surprising that PtdIns(4,5)P2 derangements influence actin organization. What is intriguing is that it is only the tip actin microfilament network that is disrupted, arguing for a specific coupling of AtSfh1p function to a PtdIns(4,5)P2 pool that regulates this specific component of the actin cytoskeleton. In contrast to the specific actin effects in Atsfh−/− root hairs, a manifest disorganization of the microtubule network is recorded. There is selectivity to this defect as well, however. While root hair microtubules are disorganized, the Atsfh−/− cells from which root hairs emanate exhibit well-formed microtubule cables (Vincent et al., 2005).
The mechanisms for spatial control of cytosolic Ca++ signaling are also responsive to AtSfh1p-mediate PtdIns(4,5)P2 synthesis and organization (Vincent et al., 2005). A tip-directed cytoplasmic Ca++ gradient is a signature feature of growing root hairs (Wymer et al., 1997), and Atsfh1−/− root hairs are manifestly defective in establishing/maintaining this gradient. Rather, the mutant root hairs elaborate precocious regions of high cytoplasmic Ca++ (∼ 1mM) with a significant, and often total, collapse of the tip-directed gradient (Vincent et al., 2005). Scanning ion-selective electrode technique measurements demonstrate that the areas of high cytosolic Ca++ observed in both wild-type and Atsfh1−/− root hairs coincide with areas of active Ca++ influx from the extracellular milieu, and not with localized Ca++ release from intracellular stores. In wild-type root hairs, those inward gradients achieve a flux of ∼ 4pmol Ca++/cm2/sec, and those regions of high flux are tightly concentrated at the tip plasma membrane. In Atsfh1−/− root hairs those inward gradients are as robust (∼ 4-8pmol Ca++/cm2/sec), but are randomly distributed along the plasma membrane (Vincent et al., 2005). We propose this spatial dysregulation of Ca++ entry reflects isotropic deposition of secretory cargo (such as Ca++ channels) into the Atsfh1−/− root hair plasma membrane. In wild-type root hairs, polarized deposition of such cargo results in tip-directed ion gradients.
The functional principles gleaned from analyses of AtSfh1p will likely apply to many (if not all) Sec14-nodulin proteins, thereby identifying these proteins as a novel class of polarity regulators. In this regard, Sec14-nodulin proteins offer interesting possibilities for a lipid signaling nanoreactor that couples phosphoinositide synthesis with spatial organization of the product (Ile et al., 2006). The spatial organization principle may reside in the nodulin domain that exhibits a highly basic C-terminal tail that exhibits features common to high affinity PtdIns(4,5)P2-binding motifs. This raises an intriguing scenario where the PtdIns(4,5)P2 pool whose synthesis is promoted by the Sec14-domain of AtSfh1p is in turn ‘sequestered’ by electrostatic interactions with the AtSfh1p nodulin domain – thereby imposing a non-random distribution of a specific PtdIns(4,5)P2 pool in the root hair plasma membrane.
Multidomain Sec14-like proteins; the Sec14-GOLD proteins
A particularly interesting set of eukaryotic Sec14p-like proteins is represented by the conserved family of Sec14- GOLD proteins. The GOLD (Golgi dynamic) domain is defined on the basis of sophisticated bioinformatics analyses that identify a homology of the GOLD domain to the luminal portion of the p24 protein superfamily of secretory cargo receptors (Anantharaman et al., 2002; Springer et al., 2000). Given the role of Sec14p in regulating membrane trafficking, and the role of p24 proteins in cargo sorting, the conserved physical linkage of Sec14p and GOLD domains must be of functional consequence. The Sec14p-like proteins represent one family of a total of six distinct families of GOLD-domain proteins. For Sec14-GOLD proteins the Sec14 domain represents an N-terminal lipid binding domain while the GOLD domain is the C-terminal domain. Mammals express five Sec14-GOLD proteins (designated SEC14L1 – SEC14L5) while Arabidopsis expresses six (designated PATL1-PATL6; Figure 5). There is essentially no information regarding the in vivo functions of Sec14-GOLD proteins. One mammalian Sec14-GOLD protein (SEC14L3, also termed p45) has an enigmatic intrinsic GTPase activity and is reported to be a secreted PtdIns(3,4,5)P3-binding protein (Merkulova et al., 2005). SEC14L3 has no obvious N-terminal signal peptide, suggesting hat secretion to the extracellular milieu must occur via some non-conventional mechanism. Why cells would secrete a PtdIns(3,4,5)P3-binding protein with GTPase activity is completely unclear.
Fig. 5.
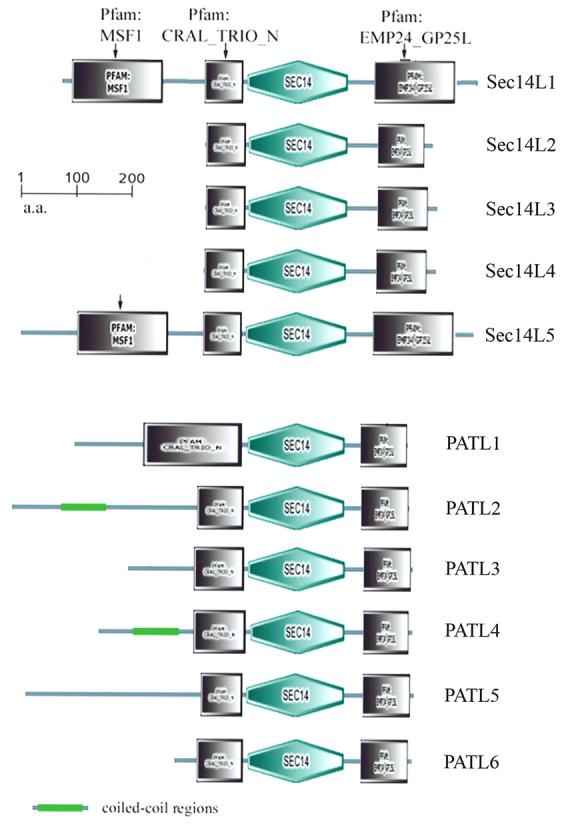
The Sec14-GOLD protein family in mammals and Arabidopsis. Schematic representation of the mammalian and Arabidopsis Sec14-GOLD proteins (top and bottom half of the Figure, respectively). Domains of interest are identified.
The six Arabidopsis Sec14p-GOLD proteins are referred to as patellins (Peterman et. al., 2004). Biochemical experiments suggest that one of the patellins, PATL1, is a phosphoinositide binding protein, but the significance of this binding is not yet known. PATL1 itself is suggested to be involved in formation and maturation of the cell plate during the late telophase stage of cytokinesis (Peterman et al., 2004), but this hypothesis is based on indirect evidence. The conservation of Sec14-GOLD proteins across the higher Eukaryota suggests an important physiological function for these proteins, and the study of these proteins promises to be a fruitful arena for future research. As with the Sec14-nodulin proteins, the Sec14-GOLD proteins also hold the promise of simultaneously regulating phosphoinsitide synthesis and organization. The PATL1 C-terminal GOLD domain exhibits a 551KX10K/RK3MQ2-3YR573 motif that resembles the known PtdIns(4,5)P2 binding motifs of AP160 and U2-adaptin (Peterman et al., 2004).
Summary
The diversity of lipid species in biological membranes testifies to the multiple roles of these molecules as structural units, precursors to second messengers, as scaffolding units that impose spatial and temporal regulation on assembly of proteins, and as regulators of the catalytic activities of proteins. Such diverse lipid functions must be appropriately coordinated so that these can be specifically and appropriately coupled to dedicated biological processes. Evidence from multiple sources is building towards a concept where Sec14-like PITPs are specific components of lipid metabolic nanoreactors and, in this capacity, help impose a functional specification of lipid signaling pools.
Acknowledgements
This work was supported by grants GM44530 and NS37723 to VAB from the National Institutes of Health. KT is supported by a Molecular Mycology and Pathogenesis Training Program Postdoctoral Traineeship from the National Institutes of Health (5T32-AI052080-04). We thank Kristina Ile and Gabriel Schaaf for helpful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achleitner G, Gaigg B, Krasser A, Kainersdorfer E, Kohlwein S, Perktold A, Zellnig G, Daum G. Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur J Biochem. 1999;264:545–53. doi: 10.1046/j.1432-1327.1999.00658.x. [DOI] [PubMed] [Google Scholar]
- Anantharaman V, Aravind L. The GOLD domain, a novel protein module involved in Golgi function and secretion. Genome Biol. 2002;3(5):0023. doi: 10.1186/gb-2002-3-5-research0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya A, Emr SD. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev Cell. 2002;2:593–605. doi: 10.1016/s1534-5807(02)00168-5. [DOI] [PubMed] [Google Scholar]
- Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–2. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Cha MK, Hong SK, Oh YM, Kim IH. The protein interaction of Saccharomyces cerevisiae cytoplasmic thiol peroxidase II with Sfh2p and its in vivo function. J. Biol. Chem. 2003;278:34952–8. doi: 10.1074/jbc.M301819200. [DOI] [PubMed] [Google Scholar]
- Cleves AE, McGee TP, Bankaitis VA. Phospholipid transfer proteins: a biological debut. Trends in Cell Biol. 1991a;1:30–4. doi: 10.1016/0962-8924(91)90067-j. [DOI] [PubMed] [Google Scholar]
- Cleves AE, McGee TP, Whitters EA, Champion KM, Aitken JR, Dowhan W, Goebl M, Bankaitis VA. Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell. 1991b;64:789–800. doi: 10.1016/0092-8674(91)90508-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleves AE, Novick PJ, Bankaitis VA. Mutations in the SAC1 gene suppress defects in yeast Golgi and yeast actin function. J Cell Biol. 1989;109:2939–50. doi: 10.1083/jcb.109.6.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MKY, Bankaitis VA. Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J. 1996;15:6447–59. [PMC free article] [PubMed] [Google Scholar]
- Guo S, Stolz LE, Lemrow S, York JD. SAC1-like domains of yeast SAC1, INP52 and INP53, and human synaptojanin encode polyphosphoinositide phosphatases. J Biol Chem. 1999;274:12990–95. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald D. Direct involvement of phosphatidylinositol-4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:34294–301. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- Henneberry AL, Lagace TA, Ridgway ND, McMaster CR. Phosphatidylcholine synthesis influences the diacylglycerol homeostasis required for Sec14p-dependent Golgi function and cell growth. Mol Biol Cell. 2001;12:511–520. doi: 10.1091/mbc.12.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts RP, Topalof L, Bankaitis VA. Lipid metabolism and regulation of membrane trafficking. Traffic. 2000;3:195–202. doi: 10.1034/j.1600-0854.2000.010301.x. [DOI] [PubMed] [Google Scholar]
- Ile KE, Schaaf G, Bankaitis VA. Phosphatidylinositol transfer proteins and cellular nanoreactors for lipid signaling. Nature Chem Biol. 2006 doi: 10.1038/nchembio835. In Press. [DOI] [PubMed] [Google Scholar]
- Im YJ, Raychaudhuri S, Prinz WA, Hurley JH. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature. 2005;437:154–8. doi: 10.1038/nature03923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapranov P, de Bruijn FJ, Szczyglowski K. Novel, highly expressed late nodulin gene (LjNOD16) from Lotus japonicus. Plant Physiol. 1997;113:1081–90. doi: 10.1104/pp.113.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapranov P, Routt SM, Bankaitis VA, de Bruijn FJ, Szczyglowski K. Novel developmental regulation of phosphatidylinositol transfer protein expression in nitrogen-fixing root nodules of the flowering plant Lotus japonicus. Plant Cell. 2001;13:1369–82. doi: 10.1105/tpc.13.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns BG, Alb JG, Jr, Bankaitis VA. Phosphatidylinositol transfer proteins: The long and winding road to function. Trends in Cell Biology. 1998a;8:276–82. doi: 10.1016/s0962-8924(98)01281-1. [DOI] [PubMed] [Google Scholar]
- Kearns BG, McGee TP, Mayinger P, Gedvilaite A, Phillips SE, Kagiwada S, Bankaitis VA. An essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature. 1997;387:101–5. doi: 10.1038/387101a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns MA, Monks DE, Fang M, Rivas MP, Courtney PD, Chen J, Prestwich GD, Theibert AB, Dewey RE, Bankaitis VA. Novel developmentally regulated phosphoinositide binding proteins from soybean whose expression bypasses the requirement for an essential phosphatidylinositol transfer protein in yeast. EMBO J. 1998b;17:4004–17. doi: 10.1093/emboj/17.14.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SM, Poon PP, Singer RA, Johnston GC, Spang A. The ArfGAP Glo3 is required for the generation of COPI vesicles. Mol Biol Cell. 2004;15:4064–72. doi: 10.1091/mbc.E04-04-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rivas MP, Fang M, Marchena J, Mehrotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA. Analysis of oxysterol binding protein homolog Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol. 2002;157:63–77. doi: 10.1083/jcb.200201037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Routt S, Xie Z, Cui X, Fang M, Kearns MA, Bard M, Kirsch D, Bankaitis VA. Identification of a novel family of nonclassical yeast PITPs whose function modulates activation of phospholipase D and Sec14p-independent cell growth. Mol Biol Cell. 2000;11:1989–2005. doi: 10.1091/mbc.11.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh BJ, Mastronarde DN, Buttle KF, Howell KE, McIntosh JR. Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proc Natl Acad Sci U. S. A. 2001;98:2399–406. doi: 10.1073/pnas.051631998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee TP, Skinner HB, Whitters EA, Henry SA, Bankaitis VA. A phosphatidylinositol transfer protein controls the phosphatidylcholine content of yeast Golgi membranes. J Cell Biol. 1994;124:273–87. doi: 10.1083/jcb.124.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkulova M, Huynh H, Radchenko V, Saito K, Lipkin V, Shuvaeva T, Mustelin T. Secretion of the mammalian Sec14p-like phosphoinositide-binding p45 protein. FEBS J. 2005;272:5595–605. doi: 10.1111/j.1742-4658.2005.04955.x. [DOI] [PubMed] [Google Scholar]
- Min KC, Kovall RA, Hendrickson WA. Crystal structure of α-tocopherol transfer protein bound to its ligand: Implications for ataxia with vitamin E deficiency. Proc Natl Acad Sci. 2003;100:14713–18. doi: 10.1073/pnas.2136684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto Y, Kearns BG, Wenk MR, Chen H, Mori K, Alb JG, Jr, De Camilli P, Bankaitis VA. Functional characterization of a mammalian Sac1 and mutants exhibiting substrate specific defects in phosphoinositide phosphatase activity. J Biol Chem. 2000;275:14446–56. doi: 10.1074/jbc.M003923200. [DOI] [PubMed] [Google Scholar]
- Peterman TK, Ohol YM, McReynolds LJ, Luna EJ. Patellin1, a novel Sec14-like protein, localizes to the cell plate and binds phosphoinositides. Plant Physiol. 2004;136:3080–94. doi: 10.1104/pp.104.045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S, Sha B, Topalof L, Xie Z, Alb J, Clenchin V, Swigart P, Cockcroft S, Luo M, Martin T, Bankaitis V. Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Molecular Cell. 1999;4:187–97. doi: 10.1016/s1097-2765(00)80366-4. [DOI] [PubMed] [Google Scholar]
- Phillips SE, Vincent P, Rizzieri K, Schaaf G, Gaucher EA, Bankaitis VA. The diverse biological functions of phosphatidylinositol transfer proteins in eukaryotes. Crit Rev in Bioch & Mol Biol. 2006;41:1–28. doi: 10.1080/10409230500519573. [DOI] [PubMed] [Google Scholar]
- Pichler H, Gaigg B, Hrastnik C, Achleitner G, Kohlwein SD, Zellnig G, Perktold A, Daum G. A subfraction of the yeast endoplasmic reticulum associates with the plasma membrane and has a high capacity to synthesize lipids. Eur J Biochem. 2001;268:2351–61. doi: 10.1046/j.1432-1327.2001.02116.x. [DOI] [PubMed] [Google Scholar]
- Rivas MP, Kearns BG, Xie Z, Guo S, Sekar MC, Hosaka K, Kagiwada S, York JD, Bankaitis VA. Relationship between altered phospholipid metabolism, DAG, ‘bypass Sec14p’, and the inositol auxotrophy of yeast sac1 mutants. Mol Biol Cell. 1999;10:2235–50. doi: 10.1091/mbc.10.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routt SM, Ryan MM, Tyeryar K, Rizzieri KE, Mousley C, Roumanie O, Brennwald PJ, Bankaitis VA. Nonclassical PITPs activate phospholipase D via the Stt4p PtdIns-4-kinase and modulate function of late stages of exocytosis in vegetative yeast. Traffic. 2005;6:1157–72. doi: 10.1111/j.1600-0854.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- Schnabl M, Oskolkova OV, Holič R, Brežná B, Pilcher H, Zágoršek M, Kohlwein SD, Paltauf F, Daum G, Griač P. Subcellular localization of yeast Sec14 homologues and their involvement in regulation of phospholipids turnover. Eur J Biochem. 2003;270:3133–45. doi: 10.1046/j.1432-1033.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- Sha B, Phillips SE, Bankaitis VA, Luo M. Crystal structure of the Saccharomyces cerevisiae phosphatidylinositol transfer protein Sec14p. Nature. 1998;391:506–10. doi: 10.1038/35179. [DOI] [PubMed] [Google Scholar]
- Shiao YJ, Lupo G, Vance JE. Evidence that phosphatidylserine is imported into mitochondria via a mitochondrial-associated membrane and that the majority of mitochondrial phosphatidylethanolamine is derived from decarboxylation of phosphatidylserine. J Biol Chem. 1995;270:11190–98. doi: 10.1074/jbc.270.19.11190. [DOI] [PubMed] [Google Scholar]
- Skinner HB, McGee TP, McMaster CR, Fry MR, Bell RM, Bankaitis VA. The Saccharomyces cerevisiae phosphatidylinositol transfer protein effects a ligand-dependent inhibition of choline-phosphate cytidylyltransferase activity. Proc Natl Acad Sci U.S.A. 1995;92:112–6. doi: 10.1073/pnas.92.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova T, Chadwick TG, MacArthur R, Poluekov O, Song L, Ryan M, Schaaf G, Bankaitis VA. The chemistry of phospholipid binding by the Saccharomyces cerevisiae phosphatidylinositol transfer protein Sec14p as determined by electron paramagnetic resonance spectroscopy. J Biol Chem. 2006;281 doi: 10.1074/jbc.M603054200. In Press; published September 22, 2006 as doi:10.1074/jbc.M603054200. [DOI] [PubMed] [Google Scholar]
- Springer S, Chen E, Duden R, Marzioch M, Rowley A, Hamamoto S, Merchant S, Schekman R. The p24 proteins are not essential for vesicular transport in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2000;97:4034–39. doi: 10.1073/pnas.070044097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker A, Tomizaki T, Schulze-Briese C, Baumann U. Crystal structure of the human supernatant protein factor. Structure. 2002;10:1533–40. doi: 10.1016/s0969-2126(02)00884-5. [DOI] [PubMed] [Google Scholar]
- Trotter PJ, Wu W-I, Pedretti J, Yates R, Voelker DR. A genetic screen for aminophospholipid transport mutants identifies the phosphatidylinositol 4-kinase, Stt4p, as an essential component in phosphatidylserine metabolism. J Biol Chem. 1998;273:13189–96. doi: 10.1074/jbc.273.21.13189. [DOI] [PubMed] [Google Scholar]
- Vincent P, Chua M, Nogue F, Fairbrother A, Mekheel H, Xu Y, Allen N, Bibikova TN, Gilroy S, Bankaitis VA. A Sec14p-nodulin domain phosphatidylinositol transfer protein polarizes membrane growth of Arabidopsis thaliana root hairs. J Cell Biol. 2005;168:801–12. doi: 10.1083/jcb.200412074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Novick P. The yeast phosphatidylinositol-4-OH kinase Pik1 regulates secretion at the Golgi. Nature Cell Biol. 1999;1:523–5. doi: 10.1038/70319. [DOI] [PubMed] [Google Scholar]
- Whitters EA, Cleves AE, McGee TP, Skinner HB, Bankaitis VA. SAC1p is an integral membrane protein that influences the cellular requirement for phospholipid transfer protein function and inositol in yeast. J Cell Biol. 1993;122:79–94. doi: 10.1083/jcb.122.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz KWA. Phospholipid transfer proteins. Annu Rev Biochem. 1991;60:73–99. doi: 10.1146/annurev.bi.60.070191.000445. [DOI] [PubMed] [Google Scholar]
- Wu W-I, Voelker DR. Biochemistry and genetics of interorganelle aminoglycerophospholipid transport. Sem Cell and Dev Biol. 2002;13:185–95. doi: 10.1016/s1084-9521(02)00047-2. [DOI] [PubMed] [Google Scholar]
- Wu W-I, Voelker DR. Reconstitution of phosphatidylserine transport from chemically defined donor membranes to phosphatidylserine decarboxylase 2 implicates specific lipid domains in the process. J Biol Chem. 2004;279:6635–42. doi: 10.1074/jbc.M311570200. [DOI] [PubMed] [Google Scholar]
- Wu WI, Routt S, Bankaitis VA, Voelker D. A new gene involved in transport-dependent metabolism of phosphatidylserine, PSTB2/PDR17, shares sequence similarity with the gene encoding the phosphatidylinositol/ phosphatidylcholine transfer protein, Sec14p. J Biol Chem. 2000;275:14446–56. doi: 10.1074/jbc.275.19.14446. [DOI] [PubMed] [Google Scholar]
- Wymer CL, Bibikova TN, Gilroy S. Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. The Plant Journal. 1997;12:427–39. doi: 10.1046/j.1365-313x.1997.12020427.x. [DOI] [PubMed] [Google Scholar]
- Xie Z, Fang M, Bankaitis VA. Evidence for an intrinsic toxicity of phosphatidylcholine to Sec14p-dependent protein transport from the yeast Golgi complex. Mol Biol Cell. 2001;12:1117–29. doi: 10.1091/mbc.12.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa L, Marchena J, Xie Z, Li X, Poon PP, Singer R, Johnston G, Randazzo PA, Bankaitis VA. Activity of specific lipid-regulated ARFGAPs is required for Sec14p-dependent Golgi secretory function in yeast. Mol Biol Cell. 2002;13:2193–206. doi: 10.1091/mbc.01-11-0563.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JS, Lee SY, Gao M, Bourgoin S, Randazzo PA, Premont RT, Hsu VW. ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J Cell Biol. 2002;159:69–78. doi: 10.1083/jcb.200206015. [DOI] [PMC free article] [PubMed] [Google Scholar]