Abstract
Cellular transition to anaphase and mitotic exit has been linked to the loss of cyclin-dependent kinase 1 (Cdk1) kinase activity as a result of anaphase-promoting complex/cyclosome (APC/C)–dependent specific degradation of its cyclin B1 subunit. Cdk1 inhibition by roscovitine is known to induce premature mitotic exit, whereas inhibition of the APC/C-dependent degradation of cyclin B1 by MG132 induces mitotic arrest. In this study, we find that combining both drugs causes prolonged mitotic arrest in the absence of Cdk1 activity. Different Cdk1 and proteasome inhibitors produce similar results, indicating that the effect is not drug specific. We verify mitotic status by the retention of mitosis-specific markers and Cdk1 phosphorylation substrates, although cells can undergo late mitotic furrowing while still in mitosis. Overall, we conclude that continuous Cdk1 activity is not essential to maintain the mitotic state and that phosphatase activity directed at Cdk1 substrates is largely quiescent during mitosis. Furthermore, the degradation of a protein other than cyclin B1 is essential to activate a phosphatase that, in turn, enables mitotic exit.
Introduction
Cdk1 and its associated protein cyclin B1 are required for entry into and maintenance of the mitotic state in mammalian cells (Evans et al., 1983; Minshull et al., 1989; Nurse, 1990). Exit from mitosis in mammalian cells requires the inactivation of Cdk1, the protein kinase that drives the mitotic state (Murray, 2004). Inactivation follows the destruction of the Cdk1-activating subunit cyclin B1 by proteolysis (Murray et al., 1989; Hershko et al., 1991; Holloway et al., 1993), a process normally activated at metaphase by anaphase-promoting complex/cyclosome (APC/C)–driven ubiquitination (Glotzer et al., 1991; Hershko et al., 1991). Failure to degrade cyclin B1 results in constitutively active Cdk1 and indefinite arrest in mitosis (Murray et al., 1989; Ghiara et al., 1991; Gallant and Nigg, 1992; Holloway et al., 1993). As Cdk1 inactivation is not required for progression past metaphase, vertebrate cells and in vitro cell model systems can arrest either in metaphase or in later stages of mitosis in the presence of constitutively active Cdk1 (Holloway et al., 1993; Wheatley et al., 1997; Stemmann et al., 2001).
Inactivation of Cdk1 itself has been considered to be necessary and sufficient to induce a rapid exit from mitosis. Exposure of cells to specific inhibitors of Cdk1 causes rapid mitotic exit (Potapova et al., 2006). The APC/C E3 ubiquitin protein ligase processively ubiquitinates specific sequence tags (Rape et al., 2006), principally D-box (Glotzer et al., 1991) or KEN-box (Pfleger and Kirschner, 2000) motifs, in multiple target proteins in the course of mitotic exit (Peters, 2002) and targets them for proteasome destruction. The degradation of two proteins, cyclin B1 and securin, is linked to proper mitotic exit. Destruction of cyclin B1 is absolutely necessary for mitotic exit (Gallant and Nigg, 1992; Holloway et al., 1993). Although the destruction of securin is required for proper chromosome segregation, failure to destroy securin does not block mitotic exit (Zur and Brandeis, 2001).
In this study, we analyze the state of cells exposed to Cdk1 inhibitors in combination with the suppression of proteolysis and present evidence that the mitotic state (defined as the continuous presence of condensed chromosomes) of a mitotic spindle and of mitotic phosphoprotein antigens is sustained for a long period in the absence of Cdk1 activity, but only when APC/C-dependent protein degradation is simultaneously suppressed.
We find that the capacity to sustain mitotic status correlates with the persistence of phosphorylated Cdk1 substrates in the absence of Cdk1 activity. Thus, our results demonstrate that Cdk1 inactivation alone is not sufficient to induce mitotic exit. Instead, key serine/threonine protein phosphatases, which are required for mitotic exit, are largely inactive during mitosis and must be reactivated by a proteolytic event so that they, in turn, can dephosphorylate Cdk1 substrates and enable mitotic exit. Our results show an unexpected convergence of the mammalian system with yeast in which phosphatase activity is required for mitotic exit (Stegmeier and Amon, 2004).
Results
Sustained mitosis in cells when both Cdk1 activity and proteolysis are suppressed
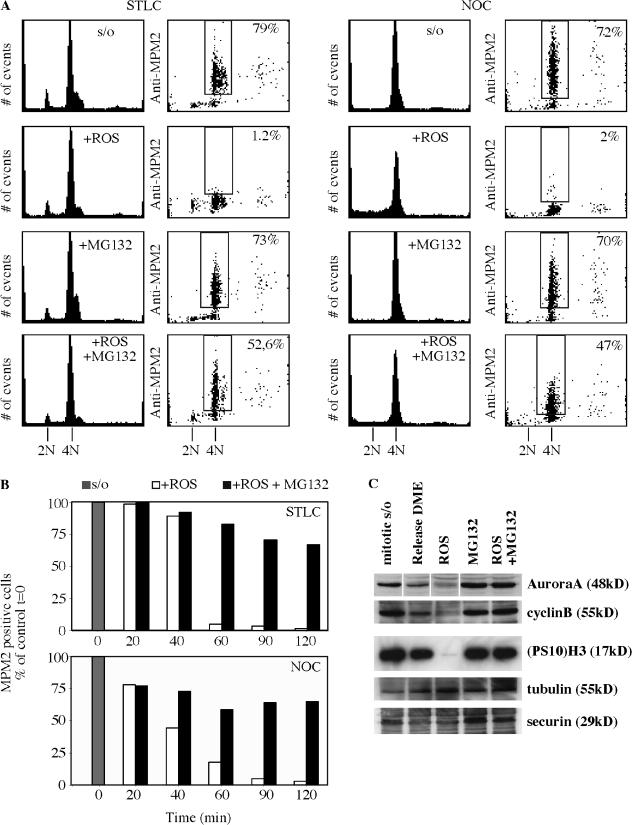
HeLa cells were collected in mitosis by exposure to S-trityl-l-cysteine (STLC), a potent and specific inhibitor of the microtubule motor protein Eg5 (Skoufias et al., 2006), or to nocodazole, an inhibitor of microtubule assembly (Zieve et al., 1980). We then tested the effect of cell exposure to the specific Cdk1 inhibitor roscovitine or to the protease inhibitor MG132. The mitotic state was determined by flow cytometric assay of the presence of MPM-2, a well-established mitosis-specific phosphoprotein substrate and mitotic marker (Davis et al., 1983; Andreassen and Margolis, 1994). As previously demonstrated (Payton et al., 2006; Vassilev et al., 2006), exposure to Cdk inhibitors such as roscovitine for 2 h induced rapid mitotic exit (Fig. 1, A and B). On the other hand, exposure to MG132 sustained the mitotic state (Brito and Rieder, 2006).
Figure 1.
Loss of Cdk1 kinase activity in the absence of proteasome activity does not lead to mitotic exit. (A) Mitotic HeLa cells were collected by selective detachment after being blocked in mitosis with 7.5 μM STLC (left) or with 0.1 μg/ml nocodazole (right) for 16 h. Cells in the continuous presence of the mitotic inhibitors were exposed to 100 μM roscovitine (ROS) or 20 μM MG132 or to both roscovitine and MG132, and samples for 2D FACScan analysis were taken 2 h after drug addition. In contrast to roscovitine treatment, cells in the presence of MG132 retained their mitotic state, as indicated by MPM2 signal. Similarly, the majority of cells coincubated with roscovitine and MG132 remained mitotic. The percentages shown indicate the cell subpopulation that was positive for MPM-2. (B) Quantitation of the percentage of cells positive for MPM-2 calculated relative to the mitotic value of cells at time 0, which was defined as the time of shake-off and addition of the various drug combinations. (C) Immunoblot analysis of various mitotic markers assayed at 2 h after shake-off of mitotic cells and addition of various drug combinations. Mitotic exit induced by roscovitine leads to the degradation of Aurora A, cyclin B1, and securin as well as loss of the mitosis-specific phosphorylation of residue S10 of H3 ((PS10)H3). In contrast, the addition of MG132, even in the presence of roscovitine, leads to stabilization of all of the proteins tested as well as retention of the phosphorylated status of H3. Release in medium alone (DME) served as a mitotic exit control.
We then tested the effect of exposing HeLa cells to the combination of roscovitine and MG132. Importantly, the presence of MG132 substantially prevented roscovitine-treated cells from losing MPM-2 phosphoproteins despite the absence of Cdk1 activity (Fig. 1). The retention of MPM-2 phosphoproteins provides evidence for continued mitotic status in the combined presence of roscovitine and MG132. Quantitation of flow cytometric data over a time course demonstrated that the percentage of mitotic cells exposed to both drugs initially diminished but then remained stable at ∼60% of the time 0 value (Fig. 1 B). The difference from the fate of cells exposed to roscovitine alone was striking. Essentially, no cells remained mitotic after 60 or 90 min of exposure to roscovitine in the continued presence of STLC or of nocodazole (Fig. 1 B).
Continued mitotic status, which is represented by the retention of MPM-2 phosphoantigen, was supported by the retention of phosphorylated histone H3. Histone H3 is phosphorylated on S10 during mitosis by Aurora kinase (Hsu et al., 2000; Giet and Glover, 2001; Crosio et al., 2002), and phosphorylated H3 has been used as a specific mitotic marker (Hendzel et al., 1997). Western blots showed that phosphorylated H3 was absent after 2 h of roscovitine exposure but was retained in the combined presence of roscovitine and MG132 (Fig. 1 C, (PS10)H3). Aurora A, securin, and cyclin B1, proteins normally degraded on mitotic exit (Peters, 2002), were largely absent after 2 h of roscovitine treatment but were retained in cells exposed to the combination of roscovitine and MG132 (Fig. 1 C).
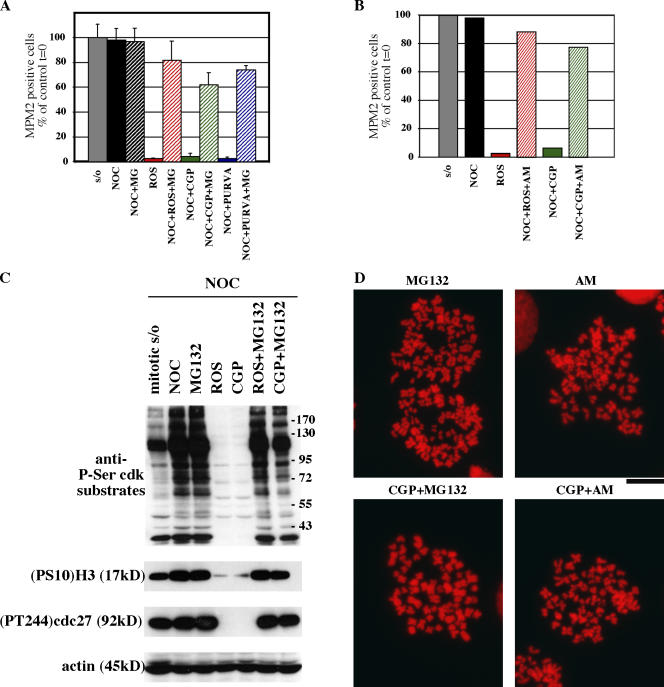
We obtained similar results after the exposure of cells to two other Cdk inhibitors, CGP74514A and purvalanol A. First, we conducted dose-response experiments to determine the minimum concentration of inhibitors capable of inducing the complete mitotic exit of nocodazole-arrested cells (unpublished data). Then, mitotic cells were exposed to the Cdk inhibitors in the presence of MG132. Results were comparable with those with roscovitine. Neither 7.5 μM CGP74514A nor 25 μM purvalanol A were able to drive mitotic exit in the presence of MG132 (Fig. 2 A). Furthermore, we also assayed the effect of substituting MG132 with another proteasome inhibitor, AM114, and obtained similar retention of the mitotic status in the absence of Cdk activity (Fig. 2 B). Western blot analysis of the extracts from treated cells showed similar patterns of the phosphorylation of Cdk substrates and of phosphorylated H3, Aurora A, and cdc27 (Fig. 2 C). Chromosome spreads confirmed the mitotic status of cells treated either with CGP74514A + MG132 or with CGP74514A + AM114 (Fig. 2 D). The different combinations of inhibitors of Cdk and proteasome argue against the possibility that our results are artifactually caused by MG132 specifically dampening the inhibitory activity of roscovitine. Additionally, we extended our 2D FACScan analysis to another human cell line, HCT116, and the results were qualitatively and quantitatively similar: compared with nocodazole-blocked cells, 85% of cells incubated with AM114 + roscovitine were MPM2 positive (unpublished data). Importantly, a nontransformed human cell line, IMR90, also remained substantially mitotic on exposure to combinations of either MG132 + roscovitine in the presence of nocodazole, AM114 + roscovitine, or AM114 + CGP74514A (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200704117/DC1). We conclude that nontransformed cells remain fully mitotic in different conditions of Cdk1 inhibition, a result comparable to that with transformed cells.
Figure 2.
Absence of mitotic exit after loss of Cdk1 kinase activity is independent of specific drugs. (A) Mitotic HeLa cells were collected by selective detachment after being blocked in mitosis with 0.1 μg/ml nocodazole for 16 h. Cells in the continuous presence of nocodazole were exposed to 20 μM MG132 (MG) for 1 h before the addition of Cdk inhibitors roscovitine (ROS; 100 μM) or CGP74514A (CGP; 7.5 μM), or cells were exposed to 25 μM purvalanol A (PURVA) for 2 h in the continuous presence of MG132. Samples for 2D FACScan analysis were taken after drug addition to determine DNA and MPM-2 content (as in Fig. 1). The percentage of 4N cells positive for MPM-2 was quantitated from FACScan and plotted relative to the mitotic value of cells at time 0, which was defined as the time of shake-off and addition of the various drug combinations. In contrast to Cdk inhibitor treatments, cells in the presence of MG132 retained their mitotic state, as indicated by MPM2 signal. Similarly, the majority of cells coincubated with Cdk inhibitors and MG132 remained mitotic. Error bars represent SD. (B) Cells were treated and analyzed as in A, but MG132 was substituted with 20 μM of the proteasome inhibitor AM114. Quantitation of the percentage of 4N cells positive for MPM-2 was calculated as in A. (C) Immunoblot analysis of various mitotic markers assayed at 2 h after shake-off of mitotic cells and addition of various drug combinations. Mitotic exit induced by Cdk inhibitors leads to loss of the mitosis-specific phosphorylation of Cdk substrates as well as the following mitotic markers: residue S10 of H3 ((PS10)H3) and residue T244 of cdc27. In contrast, the addition of MG132, even in the presence of Cdk inhibitors, leads to retention of the phosphorylated status of the proteins. Similar results were obtained for purvalanol A in place of roscovitine or CGP74514A and AM114 in place of MG132 (not depicted). (D) Chromosome spreads of representative cells in CGP74514A + MG132 or CGP74514A + AM114 show mitotic status. Results compared with MG132 or AM114 alone. DNA stain is propidium iodide. Bar, 13 μm.
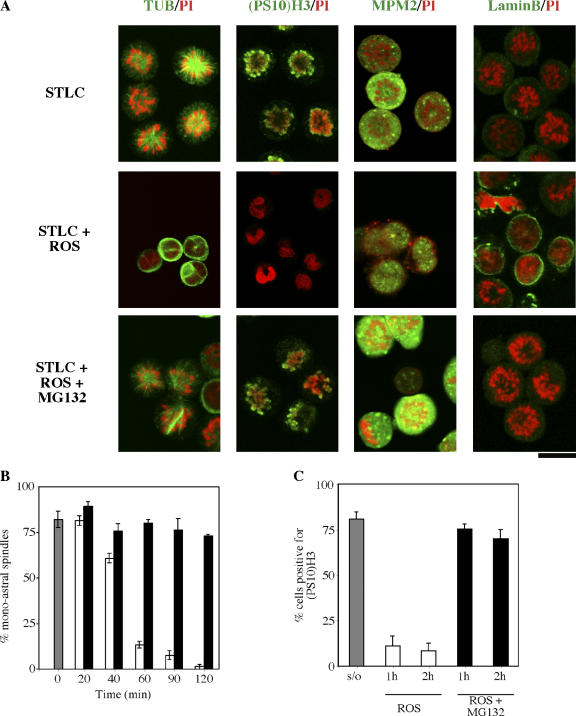
Immunofluorescence microscopy confirmed mitotic status in HeLa lacking Cdk1 activity. Fig. 3 A shows images with roscovitine + MG132. As expected, MG132 prevented the destruction of proteins normally degraded during mitotic exit. On continuous exposure to the Eg5 inhibitor STLC, control cells contained condensed chromosomes and a single aster spindle (tubulin label). The chromosomes were positive for the mitotic markers phosphohistone H3 and MPM-2. Lamin B, a marker for interphase nuclear envelopes, was dispersed, as is normal during mitosis (Gerace and Blobel, 1980). In contrast, cells treated with roscovitine for 2 h in the presence of STLC exited mitosis and, thus, lost condensed chromosomes, the monoastral spindle, histone H3 phosphorylation, and the MPM-2 phosphoantigen but had gained a lamin nuclear border (Fig. 3 A). These controls showed the status of markers in continued mitotic arrest or in mitotic exit. On exposure to the combination of roscovitine + MG132, cells appeared like the mitotic cells blocked in STLC and showed no sign of mitotic exit by any criterion despite the absence of Cdk1 activity. All Cdk and proteasome inhibitor combinations yielded similar images with respect to mitotic exit (unpublished data).
Figure 3.
Loss of Cdk1 activity in the absence of proteasome activity does not lead to mitotic exit. (A) After STLC treatment and mitotic shake-off, cells were treated with roscovitine in the absence or presence of MG132 for 2 h. Fixed cells were then stained for immunofluorescence microscopy with antitubulin, phosphorylated histone H3 ((PS10)H3), MPM-2, or anti–lamin B antibodies (green) and were counterstained with propidium iodide (PI; red). Roscovitine induced rapid mitotic exit, as evidenced by loss of the monoastral spindles, (PS10)H3, MPM-2 staining, and the assembly of nuclear lamina surrounding decondensed chromatin. However, cells treated with STLC plus roscovitine and MG132 retained monoastral spindles, (PS10)H3, and MPM-2 staining, whereas nuclear lamina were absent. These results were parallel to control cells blocked in mitosis with STLC. Microscope settings were held constant for all image acquisitions. (B and C) The percentage of cells with monoastral spindles (B) or positive for (PS10)H3 (C) were quantitated. The data represent the mean of three counts of >60 cells per count. Gray bar, cells at time 0, the time of mitotic cell selection by shake-off; white bars, cells treated with roscovitine alone; black bars, cells treated with roscovitine plus MG132. In all cases, cells were in the continuous presence of STLC. Error bars represent SD. Bar, 16.5 μm.
We quantitated the percentage of the mitotic population that retained two mitotic markers, monoastral spindles or S10 phosphorylated histone H3, during 2 h of exposure to either roscovitine alone or to the combination of roscovitine and MG132 (Fig. 3 B). For this purpose, cells were first collected by mitotic shake-off after block in STLC, which yielded ∼80% mitotic cells that were positive for each of the two markers at time 0 (Fig. 3 B). By 1 h, roscovitine exposure resulted in almost a complete loss of monoastral spindles or phosphorylated H3. In contrast, cells exposed to the combination of roscovitine and MG132 almost completely retained these markers compared with time 0. Similar results were obtained with antiphospho-S10 histone H3 (Fig. 3 C). Other Cdk and proteasome inhibitors yielded similar data (unpublished data).
To exclude the possibility that our results were caused by artifactual interference of the proteasome inhibitor with suppression of Cdk1 activity by Cdk1 inhibitors, we conducted an in vitro assay of Cdk1 histone H1 phosphorylation activity in the presence of purvalanol A or roscovitine alone, or of Cdk1 histone H1 phosphorylation activity in the combined presence of purvalanol A + MG132 or roscovitine + MG132. The result (Fig. 4) clearly showed that both purvalanol A and roscovitine substantially suppressed Cdk1 activity and that the further addition of MG132 did not interfere with Cdk1 inhibition. All drugs were assayed in vitro at the same concentrations that were effective in cells.
Figure 4.
Cdk1 inhibition by purvalanol A and roscovitine but not MG132. Immunoprecipitates pulled down with Cdk1 antibody from nocodazole-arrested HeLa cell lysates were used for in vitro kinase assays. (top) 25 μM purvalanol A or 100 μM roscovitine or the combination of purvalanol A or roscovitine with MG132 was added to the kinase assay cocktail and incubated with the immunoprecipitates for 30 min at 30°C in the presence of [32P]ATP. (bottom) Immunoblot of Cdk1 immunoprecipitates (∼10% input) cross-blotted using anti-Cdk1 antibody to demonstrate equal lane loading.
Nondegradable cyclin B1 and securin do not prevent mitotic exit in the absence of Cdk1 activity
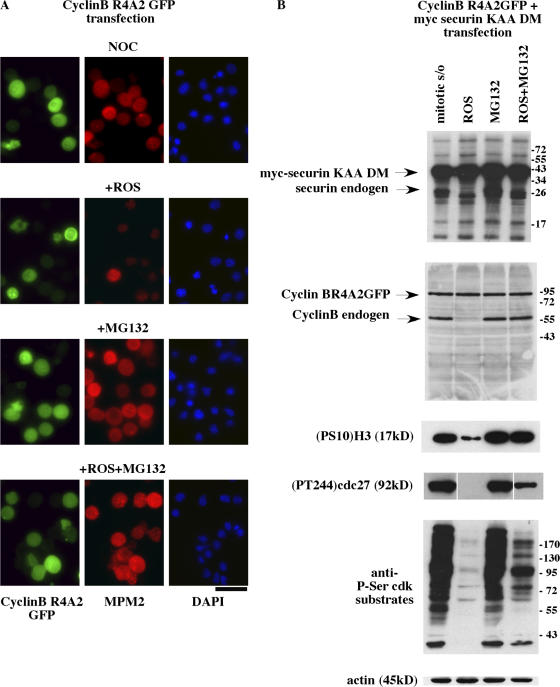
The prevention of mitotic exit using the combination of roscovitine, purvalanol, or CGP74514A with either MG132 or AM114 suggests that the suppression of degradation of a key protein protects the cell from mitotic exit in the absence of Cdk1 activity. To confirm that retention of mitotic status was not dictated by the continued presence of cyclin B1 or securin, we assayed the effect of retention of these two proteins, whose degradation is known to be essential for proper mitotic exit. For this assay, we expressed nondegradable mutants (cyclin B1 R4A2 and securin KEN DM [destruction box mutant]) of the two proteins using either single- or double-transfection protocols. Transfection with cyclin B R4A2 showed that the presence of nondegradable cyclin B was not sufficient to maintain mitosis because in the presence of roscovitine, expressing cells were negative for MPM-2, as was the case in nontransfected controls (Fig. 5 A). In contrast, transfected (GFP-positive cells) and nontransfected cells both remained positive for MPM-2 in the presence of roscovitine + MG132 after 2 h (Fig. 5 A).
Figure 5.
Degradation of cyclin B1 and securin is not necessary for mitotic exit in the absence of Cdk1 activity. (A and B) HeLa cells were transiently transfected with cDNA coding nondegradable cyclin B1 (R4A2GFP) alone (A) or both cDNA coding nondegradable cyclin B1 (R4A2GFP) and nondegradable securin (KEN-box mutant myc-securin-KAA-DM; B). At 30 h after transfection, cells were synchronized in mitosis by 16-h exposure to nocodazole. After shake-off, mitotic cells were maintained in the continuous presence of nocodazole with either roscovitine (ROS), MG132, or both roscovitine + MG132. (A) Immunofluorescence shows that nondegradable cyclin B is not sufficient to maintain mitotic status in the presence of roscovitine because GFP-positive cells are negative for MPM-2 staining. Both GFP-positive and -negative cells are MPM-2 positive after mitotic shake-off in MG132 and in roscovitine + MG132. (B) Immunoblot analysis of cellular extracts from double-transfected cells shows that in the presence of roscovitine, endogenous cyclin B1 and securin were degraded, whereas the ectopically expressed nondegradable proteins were not. Despite the continued presence of cyclin B or securin, exposure to roscovitine induced mitotic exit followed by the loss of (PS10)H3, (PT244)cdc27, and Cdk phosphoprotein substrates. In contrast, in cells treated with MG132 alone or with MG132 plus roscovitine, there was no loss of these markers, confirming the continued mitotic status of these cells. Bar, 44.5 μm.
After double transfection, cells retained their mitotic status in the presence of MG132 + roscovitine, as indicated by Western blot analysis of the mitotic-specific phosphorylation of Cdk substrates as well as by the presence of mitosis-specific markers, phosphorylated H3 and cdc27 (Fig. 5 B). We found that neither cyclin B1 R4A2 nor securin KEN DM was degraded on exposure of mitotic cells to roscovitine (Fig. 5 B). Nonetheless, cells rapidly exited mitosis, as assayed by the loss of S10 phosphohistone H3, PT244cdc27, and Cdk phosphosubstrates. As before, the combination of roscovitine and MG132 prevented mitotic exit. Endogenous cyclin B1 and securin acted as internal controls in these experiments and degraded during mitotic exit in the presence of roscovitine (Fig. 5 B). Similar Western blot results were obtained after single transfection with either cyclin B1 R4A2 or securin KEN DM alone (unpublished data).
Late mitotic events in the absence of Cdk1 and proteasome activity
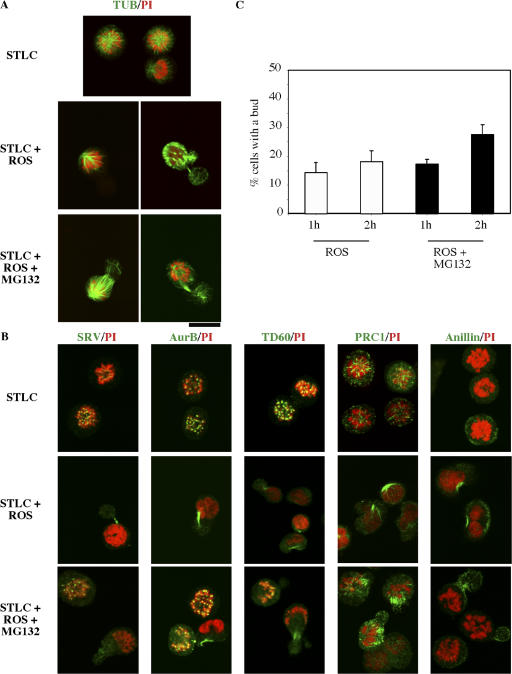
The combined presence of a Cdk1 inhibitor and a protease inhibitor induced mitotic cells to proceed to cell cleavage (Potapova et al., 2006). We have carefully observed the status of cells blocked by a combination of roscovitine and MG132, and we have found the initiation of monoastral furrowing in a substantial subpopulation of cells. This furrowing event was essentially the same as that previously observed in cells treated with monastrol (another inhibitor of Eg5) and forced to exit mitosis by suppression of the spindle assembly checkpoint (Canman et al., 2003). Indeed, cells frequently formed what we interpreted as a bud, creating a small daughter cell that contained no chromatin (Fig. 6 A). Such bud formation occurred with approximately equal frequency in cells treated with roscovitine alone or with the combination of roscovitine and MG132. The difference in outcome was that treatment with roscovitine alone caused the loss of mitotic chromosomes, whereas budded cells treated with both roscovitine and MG132 retained mitotic chromosomes.
Figure 6.
Cells with monoastral spindles cleave without mitotic exit after loss of Cdk1 activity in the presence of proteasome inhibitor. (A and B) HeLa cells collected in mitosis by 16-h exposure to STLC followed by mitotic shake-off were maintained in STLC and treated with roscovitine in the presence or absence of MG132 for 2 h. Cells were then fixed and stained for microscopy with antibodies to tubulin (A) or to the cleavage furrow–associated proteins survivin, Aurora B, TD60, PRC1, or anillin (B). Exposure of STLC-treated cells to roscovitine induced a furrowing event, characteristically with formation of a bud devoid of chromatin. The passenger proteins survivin, Aurora B, and TD60 associated with the furrow, as did PRC1 and anillin. (C) The percentage of cells with a bud was quantitated after 1 or 2 h of exposure to drugs. The data represent the mean of three counts of >60 cells per count. White bars, cells treated with roscovitine; black bars, cells treated with both roscovitine and MG132. All cells were in the continuous presence of STLC. Error bars represent SD. Bar, 16.5 μm.
The budding was a true furrowing event, as the passenger proteins survivin (Skoufias et al., 2000), Aurora B (Adams et al., 2000), and TD60 (Mollinari et al., 2003) relocalized to the neck of the bud both in cells treated with roscovitine alone or with the combination of roscovitine and MG132 (Fig. 6 B). Furthermore, PRC1 and anillin, two proteins that localize to the furrow and are critical to cell cleavage (Jiang et al., 1998; Oegema et al., 2000; Mollinari et al., 2002, 2003), were also present at the bud necks (Fig. 6 B). The percentage of cells that exhibited budding is summarized (Fig. 6 C). We interpret these results as indicating that furrowing occurs in the absence of Cdk1 activity and in the absence of proteolysis. However, such furrowing occurs in the absence of mitotic exit.
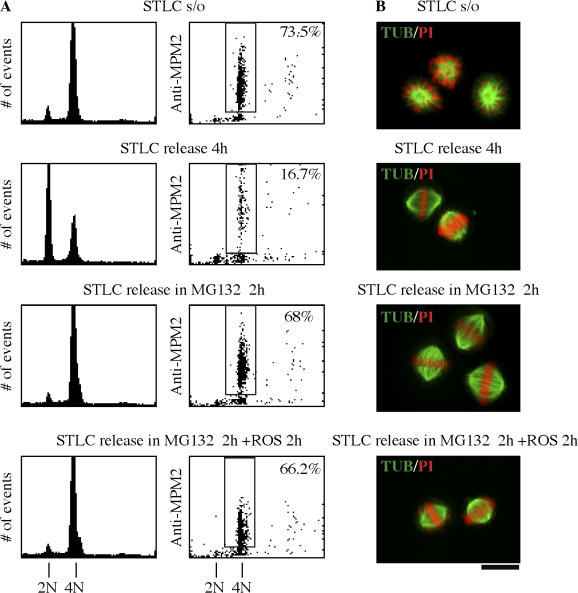
Because monoastral cells proceeded to furrow in the presence of roscovitine and MG132, we next addressed the fate of cells released from STLC block in the presence of roscovitine and MG132. The question was whether cells without Cdk1 activity would proceed through the normal stages of mitosis or would proceed directly to furrowing in the absence of Cdk1 activity. For this experiment, HeLa mitotic cells were collected by shake-off after 18 h of block in STLC and were released from STLC in the presence of MG132 alone or roscovitine + MG132. When cells were released without further treatment, <17% remained mitotic at 4 h as determined by FACScan analysis (Fig. 7 A). In contrast, release into MG132 for 2 h yielded 68% in mitosis compared with 73.5% in the initial mitotic population. The same result was obtained when cells were released into MG132 for 2 h and treated with a combination of roscovitine and MG132 for a further 2 h. Despite the absence of Cdk1 activity, there was no further loss of mitotic population (66.2% at 4 h). Observed by immunofluorescence microscopy, the population of cells treated with the combination of roscovitine and MG132 was largely prometaphase (bipolar spindles with most chromosomes aligned to the metaphase plate but with a few chromosomes misaligned) or metaphase (bipolar spindles with all chromosomes aligned to the metaphase plate), as shown in Fig. 7 B. Thus, the absence of Cdk1 activity did not prevent cells from maintaining a bipolar spindle and a metaphase chromosome array. Furthermore, no cells treated in this manner proceeded to furrow in this time frame, but all remained in early mitosis in the absence of Cdk1 activity (Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200704117/DC1). It is of interest to note that coincubation with roscovitine and MG132 caused the reproducible loss of metaphase cells by reversion to prometaphase (Fig. S2 A).
Figure 7.
The absence of Cdk1 activity does not prevent bipolar spindle formation and metaphase chromosome alignment on release from STLC. (A) After 16-h STLC block and mitotic shake-off, cells were released from STLC by three washes in drug-free medium and were treated with MG132 for 2 h followed by 2-h coincubation with both roscovitine and MG132. Samples were analyzed by 2D FACScan as in Fig 1. Controls were released from STLC for 4 h with no further drug treatment. Percentages shown indicate the subpopulations that were positive for MPM-2. (B) Confocal microscopy was performed on identically treated cells, which were stained for tubulin (green) and DNA with propidium iodide (PI; red). The combined FACScan and microscopy data indicate that controls released from STLC form normal bipolar spindles and exit normally from mitosis. In contrast, cells released from STLC in MG132 remain mitotic but within 2 h have normal mitotic spindles with chromosomes aligned to a normal metaphase plate. After an additional 2 h without Cdk1 activity (MG132 plus roscovitine), cells remained mitotic with normal bipolar spindles and metaphase chromosome alignment. Bar, 16.5 μm.
We conducted a parallel analysis of cells after release from STLC into MG132 followed by exposure to Cdk1 inhibitor CGP74514A. Immunofluorescence yielded metaphase mitotic images similar to those seen with roscovitine (Fig. S2 B). As with roscovitine + MG132, coexposure of CGP74514A with MG132 yielded largely bipolar spindles, but with a small increase in monopolar cells (Fig. S2 C). These data suggest that continued Cdk1 activity may be required to maintain the metaphase alignment of chromosomes.
Phosphorylation status of most Cdk1 substrates is retained when both Cdk1 and proteasome activities are inhibited
Maintenance of the mitotic state in the absence of Cdk1 activity might depend on the retention of phosphorylated Cdk1 substrates during combined Cdk1 suppression + MG132 treatment. In Fig. 2 C, using an antibody specific for Cdk1 substrate consensus motif phosphopeptides, we showed that the phosphorylation status of Cdk1 substrates (Stukenberg et al., 1997) was indeed retained in cells exposed to the combination of roscovitine + MG132 or CGP74514A + MG132. Western blots showed that mitotic cells were highly positive for a large number of Cdk1 phosphoepitopes and that these substrates were absent after 2 h of Cdk1 inhibitor exposure (Fig. 2 C).
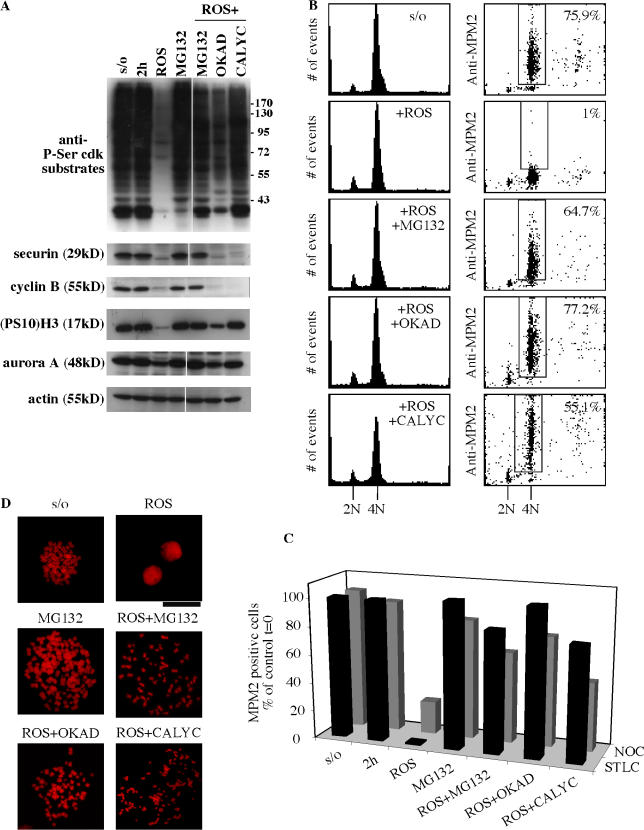
These results suggested that protein phosphatases were not acting on Cdk1 substrates in the absence of Cdk1 activity. As this offered a possible mechanism for sustained mitotic status without Cdk1, we directly assayed for the effect of phosphatase inhibition in cells treated with roscovitine and found that the dephosphorylation of Cdk1 substrates was indeed suppressed in mitotic cells exposed to the combination of roscovitine plus either okadaic acid or calyculin A (Fig. 8 A), two specific inhibitors of PP1 and PP2A protein phosphatases (Bialojan and Takai, 1988; Ishihara et al., 1989; Cohen et al., 1990). These results correlated with FACScan data showing retention of the mitotic marker MPM-2 in STLC-treated cells (Fig. 8 B). Results quantitated for cells collected in mitosis either with STLC or with nocodazole (Fig. 8 C) show substantial mitotic retention with roscovitine + either okadaic acid or calyculin A. In comparison, the inhibition of cdc25, a mitotic protein phosphatase (for review see Trinkle-Mulcahy and Lamond, 2006), was without effect on mitotic exit of roscovitine-treated cells, nor did its suppression protect cells from the dephosphorylation of Cdk1 substrates (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200704117/DC1).
Figure 8.
Phosphatase activity is required for mitotic exit in the absence of Cdk1 activity. (A) Western blot analysis of mitotic markers in extracts. Cells were collected in mitosis by STLC block (16 h) and shake-off and were exposed to different conditions for 2 h as indicated. All cells were maintained in STLC during this time course. Cells were exposed to 100 μM roscovitine (ROS), 20 μM MG132, both roscovitine and MG132, both roscovitine and 0.5 μM okadaic acid (OKAD), or to both roscovitine and 30 nM calyculin A (CALYC). Controls were cells in the continuous presence of STLC (2 h) or STLC + MG132 (MG132). S/O indicates the time 0 shake-off sample. Cell extracts were probed with a polyclonal antibody specific for Cdk substrates phosphorylated on serine (anti P-Ser Cdk substrates), with antibody specific for histone H3 phosphorylated on Ser10 ((PS10)H3), and were also probed with securin, cyclin B1, and aurora A antibodies. Anti-actin was used as loading control. (B) FACScan analysis of mitotic HeLa cells collected by shake-off after being blocked in mitosis with 7.5 μM STLC for 16 h. Cells in the continuous presence of STLC were exposed to 100 μM roscovitine (ROS), 20 μM MG132, both roscovitine and MG132, both roscovitine and 0.5 μM okadaic acid (OKAD), or both roscovitine and 30 nM calyculin A (CALYC). Samples for 2D FACScan analysis were taken 2 h after the collection of mitotic cells by shake-off. Percentages shown indicate the subpopulations that were positive for MPM-2. Parallel results were obtained with nocodazole-treated cells. (C) Comparison of results with nocodazole or with STLC block. Quantitation of the percentage of cells positive for MPM2 includes results as shown in B plus a parallel set of data from nocodazole-arrested cells that were otherwise treated identically. Data demonstrate that parallel results were obtained with nocodazole or STLC-treated cells. (D) Chromosome spreads 2 h after collection by arrest with nocodazole followed by mitotic shake-off as described in A. Cells were maintained in nocodazole and exposed to various conditions after shake-off as indicated. Bar, 19.36 μm.
Chromosome spreads of cells treated with Rosc + either okadaic acid or calyculin A showed that condensed mitotic chromosomes were maintained at high levels with the combined suppression of Cdk1 and phosphatase activity (Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200704117/DC1). Representative chromosome spreads are shown in Fig. 8 D.
Condensed chromosomes were equivalently maintained with either MG132 alone or Rosc + MG132 but unlike roscovitine alone, in which the majority of cells had reformed nuclei by 2 h (Fig. S4). Interestingly, mitotic arrest with Rosc + either okadaic acid or calyculin A occurred despite the loss of cyclin B1 and securin by 2 h (Fig. 8 A). Thus, phosphatase activity did not appear to be required for activation of cyclin B1 or securin degradation by APC/C-linked proteolysis.
We conclude that phosphatase activity, possibly involving either PP1 or PP2A, is an essential prerequisite for mitotic exit after Cdk1 inactivation. Furthermore, our data suggest that MG132-sensitive proteolysis of a proteasome substrate other than cyclin B1 is required to activate this phosphatase activity.
Discussion
Maintenance of the mitotic state depends on Cdk1 activity. Loss of Cdk1 activity normally occurs at the metaphase to anaphase transition once the spindle assembly checkpoint and other mitotic checkpoints have been satisfied (Kops et al., 2005). Mitotic exit normally occurs through the cdc20-activated APC/C-dependent degradation of two key substrates, cyclin B1 and securin.
We have demonstrated that mitotic cells will remain mitotic for several hours in the absence of Cdk1 activity provided that APC/C-dependent protease activity is suppressed. Continued mitotic status in the absence of Cdk1 activity has been verified by several independent criteria: the continued presence of condensed chromosomes, the presence of a mitotic spindle, the presence of the mitosis-specific phosphoantigen markers MPM-2 and S10 of histone H3, and the presence on chromosomes of mitotic passenger proteins. Finally, and most importantly, we show that Cdk1 substrates remain phosphorylated in the absence of Cdk1 activity.
Thus, our results require a revision of the prevailing paradigm, which holds that destruction of cyclin B1, which inactivates Cdk1, is itself necessary and sufficient to induce mitotic exit. This paradigm requires that suppression of Cdk1 activity should therefore induce mitotic exit even in the absence of cyclin B1 destruction. Instead, our results show there is a pathway downstream of Cdk1 inactivation that requires both further proteolysis and phosphatase activation to complete the mitotic exit pathway.
Our results contrast with a recent study that proposed that cells exposed to both a Cdk1 inhibitor and an inhibitor of proteolysis undergo cell cleavage but remain competent to revert to mitosis when the Cdk1 inhibitor is removed (Potapova et al., 2006). The interpretation of these results as evidence for a reversible exit from mitosis dependent on the continued presence of cyclin B1 rested on the reversibility of cell cleavage and the reappearance of a rounded mitotic cell. Other markers that would confirm that mitotic exit had indeed occurred in the absence of Cdk1 activity combined with the suppression of proteolysis were not examined except phosphorylated nucleolin.
Potapova et al. (2006) suggested that mitotic exit occurred with some Cdk1 inhibitors such as purvalanol A but not with roscovitine. Importantly, our results have been obtained with three different Cdk inhibitors and with two different proteasome inhibitors, eliminating the possibility that the capacity to remain in mitosis in the absence of Cdk1 activity is dependent on a particular drug combination.
In accord with Potapova et al. (2006), we find that a portion of blocked monoastral cells undergo transient furrowing. This furrowing is accompanied by the relocation of proteins important to cytokinesis, such as the passenger proteins Aurora B, survivin, and TD60, and of PRC1 to the cell cortex along a bundle of microtubules. As a result, these cells form a bud that contains no chromatin. Such furrowing is quite similar to that previously shown to occur in cells with monoastral spindles in which the metaphase checkpoint had been suppressed by the introduction of mutant Mad2 (Canman et al., 2003). These results and previous work (Martineau et al., 1995; Wheatley et al., 1997; Stemmann et al., 2001; Niiya et al., 2005) indicate that cytokinesis is an event that is independent of, and neither synchronous nor synonymous with, mitotic exit. Furrowing can occur well after mitotic exit has occurred or, as in the case presented here, can occur in the absence of mitotic exit. However, induction to undergo furrowing may depend on the absence of Cdk1 activity.
Cells released from STLC arrest in the presence of MG132 proceed to metaphase and maintain a bipolar metaphase spindle for at least an additional 2 h after the suppression of Cdk1 activity by roscovitine. The absence of Cdk1 activity clearly does not, of itself, drive cells forward from metaphase to anaphase. It is of interest that a substantial percentage of cells revert from metaphase to prometaphase when Cdk1 activity is suppressed in MG132-treated cells (Fig. S2), whereas none are driven forward to anaphase. When chromosomes are lost from the metaphase plate, they appear to have a merotelic (both kinetochores associated with one spindle pole) kinetochore alignment (unpublished data). Therefore, it appears that continuous Cdk1 activity is required to maintain proper metaphase chromosome alignment. This apparently unique role for Cdk1 activity in maintaining bipolar kinetochore attachment has not been noted before and deserves attention.
Retention of cells in mitosis is not caused by the continued presence of either cyclin B1 or securin in the absence of Cdk1 activity, as nondegradable mutants of these proteins do not prevent mitotic exit in the absence of Cdk1 activity. As MG132 nonetheless prevents mitotic exit, the reasonable conclusion is that a protease substrate other than cyclin B1 or securin must be degraded to permit mitotic exit in the absence of Cdk1 activity. Two proteins other than cyclin B1 and securin must be degraded for mitosis to progress, but these proteins, cyclin A (den Elzen and Pines, 2001; Geley et al., 2001) and Emi1 (Guardavaccaro et al., 2003; Margottin-Goguet et al., 2003), are both eliminated very early in mitosis and are unlikely to play a role in mitotic exit.
The putative protease substrate must be involved in phosphatase activation. Cdk1 substrates remain substantially phosphorylated for hours in the combined presence of different Cdk and proteasome inhibitors, and we obtain parallel results on exposing cells to the combination of roscovitine with protein phosphatase inhibitors (okadaic acid or calyculin A).
Suppression of the PP1 and PP2A protein phosphatase families is required for entry into mitosis (Cyert and Thorner, 1989; Dohadwala et al., 1994; Wera and Hemmings, 1995; Kwon et al., 1997; Puntoni and Villa-Moruzzi, 1997), and both okadaic acid and calyculin A specifically inhibit the PP1 and PP2A protein phosphatase families (Ishihara et al., 1989). Reasonable candidates for control of mitotic exit are members of the PP1 family, as they remain suppressed in mammalian cells by phosphorylation until metaphase and can be prolonged in this suppressed state by exposure of mitotic cells to either okadaic acid or calyculin A (Kwon et al., 1997). Furthermore, microinjection of anti-PP1 antibody arrests mammalian cells at metaphase (Fernandez et al., 1992). Similarly, the two PP1 proteins in Schizosaccharomyces pombe are suppressed by cdc2 phosphorylation in mitosis, and their reactivation is required to proceed past metaphase (Ishii et al., 1996). PP1 activity is also required for mitotic exit in Aspergillus (Doonan and Morris, 1989) and Drosophila (Axton et al., 1990; Chen et al., 2007).
Our results with the combination of roscovitine and the phosphatase inhibitors okadaic acid or calyculin A indicate that phosphatase activity is required for exit from the mitotic state, presumably by the dephosphorylation of mitotic Cdk1 substrates, and, importantly, that there is little phosphatase activity evident on Cdk1 substrates in the presence of roscovitine and MG132. The continuing mitotic state, which is characterized by condensed chromosomes and by the presence of MPM-2 and S10-phosphorylated histone H3 markers, indicates that the phosphatase activity required for mitotic exit is minimal when APC/C-dependent proteolysis has been suppressed by MG132. The potential role of protease inhibition in the suppression of phosphatase-dependent mitotic exit is of substantial interest.
The key phosphatase activity must be downstream of the cdc20-driven APC/C-dependent protease activity, as cells treated with phosphatase inhibitors in the presence of roscovitine have lost both cyclin B1 and securin (Fig. 8) but remain metabolically mitotic through the absence of the phosphatase-dependent destruction of Cdk1 substrates.
In light of a potential key role for protein phosphatases in mitotic exit, it is important to note that both budding and fission yeast contain mitotic exit networks that are dependent on unique protein phosphatases of the cdc14 family (Visintin et al., 1998; Trautmann and McCollum, 2002; Stegmeier and Amon, 2004). Although mammalian cdc14A and B are functional homologues of cdc14 phosphatases in the yeast system (Vazquez-Novelle et al., 2005), suppression of cdc14A in mammalian cells does not prevent mitotic exit (Mailand et al., 2002). We do not believe that the cdc14 mechanism is likely to play an equivalent role in the pathway we describe here, as S. pombe cdc14 (Clp1/Flp1) directly inactivates cdc2 (the S. pombe homologue of Cdk1) by regulating its phosphorylation status. In this scenario, phosphatase inhibitors would not be expected to retain mitotic status in the absence of Cdk1 activity. In light of our results with combined Cdk and phosphatase inhibitors, it is interesting that in Saccharomyces cerevisiae, Cdk activity is required to activate Net1 and, thus, cdc14 in the mitotic exit pathway and that this overrides PP2A-dependent metaphase arrest (Queralt et al., 2006). It will be of substantial interest if a parallel exists between our results and the yeast pathway that uses Cdk activity and phosphatase activation for mitotic exit.
Our results strongly implicate phosphatase activity in key events in mitotic exit and protease in activating this pathway. Although it remains to be seen whether these effects directly or indirectly involve cdc14, it is nonetheless of great interest that there appears to be a convergence between the yeast systems and the mammalian system in the functional requirement for activation of protein phosphatase activity to enable mitotic exit.
In summary, we find that cells without Cdk1 activity remain mitotic by several criteria as long as there is no APC/C-dependent protease activity and that this effect is dependent on mitotic suppression of a key protein phosphatase activity. It will now be of great interest to elucidate this important pathway, to determine the protease substrate that is retaining these cells in mitosis, and to identify the protein phosphatase whose activation is apparently required for mitotic exit.
Materials and methods
Cell culture
HeLa cells were grown in DME (Invitrogen) supplemented with 10% FCS and were maintained in a humid incubator in a 5% CO2 environment at 37°C. Mitotic cells were collected by shake-off after 16-h incubation with either 0.1 μg/ml nocodazole (Sigma-Aldrich) or with 7.5 μM STLC (Novabiochem). After centrifugation at 1,000 rpm for 5 min at 37°C, cells were resuspended in media in the continuous presence of the mitotic inhibitors with either 100 μM roscovitine (Calbiochem) or a combination of roscovitine with 20 μM MG132 (Sigma-Aldrich), or cells were resuspended with 0.5 μM okadaic acid (Calbiochem) or 30 nM calyculin A (Calbiochem) for up to 2 h. Alternatively, mitotic cells were resuspended in media in the continuous presence of the mitotic inhibitors with either 7.5 μM CGP74514A (Calbiochem) or 25 μM purvalanol A (Calbiochem) or with the combination of CGP74514A or purvalanol A with either 20 μM MG132 (Sigma-Aldrich) or AM114 (Calbiochem) for up to 2 h. In experiments using CGP74514A or purvalanol A, proteasome inhibitors were added 1 h before addition of the Cdk inhibitors.
HeLa cells were transfected with either wild-type cyclin B1-GFP or the nondegradable (R42A)-cyclin B1-GFP in pCMX vector (gifts from J. Pines, Gurdon Institute, Cambridge, UK) or were transfected with the wild-type myc-securin or nondegradable securin mutant myc-securin-KAA-DM in pCS2 vector (gifts from M. Brandeis, Hebrew University, Jerusalem, Israel). Double transfection of the nondegradable (R42A)-cyclin B1-GFP and the nondegradable securin mutant myc-securin-KAA-D was also performed. Single or double plasmid transfections were performed with LipofectAMINE (Invitrogen) according to the manufacturer's protocols. Cells were blocked in mitosis with nocodazole at 30 h after transfection and treated with the various inhibitors as described in the paragraph above.
Flow cytometric analysis
Cells were analyzed by 2D flow cytometry using MPM-2 monoclonal antibody recognizing mitosis-specific phosphoepitopes (Davis et al., 1983) and propidium iodide, a marker of DNA content. Cells were fixed, incubated with MPM-2 antibodies, and labeled with FITC-conjugated anti–mouse IgG secondary antibodies (Jackson ImmunoResearch Laboratories) and propidium iodide as described previously (Andreassen et al., 2004). Data were collected with a flow cytometer (FACScan; Becton Dickinson) using propidium iodide in the first dimension and MPM-2 in the second dimension (presented as a dot plot) and were analyzed with CellQuest software (Becton Dickinson). For each sample, 10,000 events were collected, and aggregated cells were gated out.
Immunofluorescence microscopy
Mitotic cells were fixed with 2% PFA in PBS for 20 min at 37°C, permeabilized with 0.2% Triton X-100 in PBS for 3 min, stained in suspension, and spun onto coverslips at 215 g for 3 min. The following antibodies were used for indirect immunofluorescence microscopy. Monoclonal antibody to β tubulin (Sigma-Aldrich) was used at a 300-fold dilution, MPM-2 mouse monoclonal antibody (Upstate Biotechnology) was used at 1:100, and Aurora B was detected with a rabbit polyclonal antibody (Abcam) at a 500-fold dilution. PRC1 affinity-purified rabbit antibody (gift from W. Jiang, Burnham Institute of Medial Research, La Jolla, CA; Jiang et al., 1998), JH human autoimmune serum recognizing human TD60 (Andreassen et al., 1991), and rabbit polyclonal antisurvivin (Novus Biological) were all used at 500-fold dilutions. Goat anti–lamin B (Santa Cruz Biotechnology, Inc.) was used at a 300-fold dilution. Rabbit polyclonal antibody to anillin (gift from C. Field, Harvard University, Cambridge, MA) was used at a 600-fold dilution. Secondary antibodies (Jackson ImmunoResearch Laboratories), including FITC-conjugated affinity-purified goat anti–mouse, anti–rabbit, and rabbit anti–goat IgG antibodies, were used at 1:250; Cy3-conjugated affinity-purified goat anti–mouse was used at 1:400. DNA was detected by incubation with 0.2 μg/ml propidium iodide in PBS for 5 min after incubation with secondary antibodies. Samples were observed using a microscope (Optiphot; Nikon) coupled to a laser-scanning confocal apparatus (MRC-600; Bio-Rad Laboratories) using the image acquisition software COMOS (Bio-Rad Laboratories); scans were obtained using a 40× NA 1.0 oil objective (Nikon). Treated cells, which were transfected with the nondegradable (R42A)-cyclin B1-GFP, were fixed and stained in the same manner as the above samples and were finally stained with DAPI with Vectashield mounting medium (Vector Laboratories). Images were acquired with an epifluorescence microscope (BX61; Olympus) equipped with a CCD camera (Retiga-SRV; QImaging) driven by Volocity software (Improvision) with a binning of 2 using a planApo 60× NA 1.42 objective (Olympus). Figures were processed in Photoshop version 7.0 (Adobe) and assembled in CANVAS version 8.0 (Denaba Systems).
Cdk1 kinase assay
Kinase assays were performed as described previously (Andreassen and Margolis, 1994). In brief, HeLa cells were treated with 0.2 μg/ml nocodazole for 16 h, and mitotic cells were collected by shake-off, washed once with PBS, and lysed in ice-cold buffer C as described previously (Panopoulos et al., 2005) containing fresh protease inhibitors (2 μg/ml leupeptin, 20 μg/ml aprotinin, 2 μg/ml pepstatin, and 1 mM PMSF), phosphatase inhibitor (1.3 mM p-nitrophenyl phosphate), and 2 mM DTT. Mouse monoclonal anti-Cdk1 antibody (Abcam) was incubated on ice with mitotic lysate (2 μl/100 μg protein) for 30 min before the addition of protein A–Sepharose beads (Zymed Laboratories). After the addition of beads, the lysates were incubated for 5 h at 4°C, rotating end over end. Immune complexes were washed once with 600 μl buffer C and twice with kinase assay buffer (20 mM Tris, pH 7.6, and 20 mM MgCl2). A master kinase cocktail mix was prepared containing kinase assay buffer, 1 μg/ml histone H1, 0.2 μCi/μl γ-[32P]ATP, 30 μM ATP (VWR), and 1 μM DTT. Master kinase cocktail was added to separate tubes containing water (vehicle), purvalanol A (25 μM final), roscovitine (100 μM final), MG132 (25 μM final), purvalanol A and MG132 (25 μM final each), or roscovitine and MG132 (100 μM final and 25 μM final, respectively). For immunoblots, immunoprecipitated Cdk1 complexes were resolved by SDS PAGE, transferred to polyvinylidene difluoride membranes, blocked with 5% nonfat milk, and probed with anti-Cdk1 antibody (1:500 dilution; Abcam).
Chromosome spreads
To prepare chromosome spreads, cells in suspension were pelleted by centrifugation at 215 g for 2 min and were resuspended in 75 mM KCl at 37°C for 30 min. Cells were then centrifuged again (2 min at 215 g) and fixed overnight at −20°C in 75% methanol and 25% acetic acid. Spreads were then obtained by resuspension of fixed cells and centrifugation onto coverslips (Cytospin centrifuge; Thermo Scientific) at 2,800 rpm for 3 min. The chromosome spread was then washed with PBS, stained for 5 min with 0.5 μg/ml propidium iodide in PBS, washed again twice, and mounted for observation.
Immunoblotting
Cells were lysed in 50 mM Tris-HCl, pH 7.4, 250 mM NaCl, 5 mM EGTA, and 0.1% NP-40 and were supplemented with protease and phosphatase inhibitors for 30 min on ice. 20 μg of lysates was resolved on polyacrylamide gels and transferred to nitrocellulose sheets. Materials are listed with their dilutions as follows: β tubulin was detected with a mouse anti–β tubulin monoclonal antibody (Sigma-Aldrich), 1:1,000; mouse anti–cyclin B1 monoclonal antibody (clone GNS1; Santa Cruz Biotechnology, Inc.), 2,000-fold dilution; rabbit polyclonal anti–Aurora A (Cell Signaling), 1,000-fold dilution; polyclonal antisecurin antibody (gift from H. Zou, University of Texas Southwestern Medical Center at Dallas, Dallas, TX; Zou et al., 1999), 500-fold dilution; polyclonal antiactin, 10,000-fold dilution (Sigma-Aldrich); rabbit antiphospho-S10 H3 (Upstate Biotechnology), 5,000-fold dilution; mouse monoclonal anti-myc (clone 9E10; Santa Cruz Biotechnology, Inc.), 1,000-fold dilution; rabbit antiphospho-(serine)-CDK substrate antibody (Cell Signaling), 1,000-fold dilution; and rabbit antiphospho-(T244)-cdc27 antibody (Abcam), 1,000-fold dilution. Nitrocellulose sheets were then incubated with HRP-conjugated goat anti–mouse and anti–rabbit IgG secondary antibodies. Protein–antibody complex was detected by enhanced chemiluminescence (Pierce Chemical Co.).
Online supplemental material
Fig. S1 shows that nontransformed cells are retained in mitosis after loss of Cdk activity when proteasome activity is also suppressed. Fig. S2 shows that the absence of Cdk1 activity permits bipolar spindle formation and metaphase chromosome alignment on release from STLC, but many cells revert to prometaphase. Fig. S3 shows that cdc25 phosphatase activity has no effect on mitotic exit. Fig. S4 shows quantitation of treated cells for chromosomes versus interphase nuclei. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200704117/DC1.
Acknowledgments
This paper is dedicated to the memory of Arun Fotedar, a great guy.
We thank J. Pines and M. Brandeis for providing cyclin B1 and securin plasmids, respectively. We also thank H. Zou for antisecurin antibodies, W. Jiang for anti-PRC1 antibodies, and C. Field for anti-anillin antibodies.
This work was supported by the Ligue Nationale Contre le Cancer (Laboratoire Labellisé; grant EL2005.LNCC/RM1 to R.L. Margolis), the Agence Nationale de la Recherche (grant NT05-3_42614 to R.L. Margolis and D.A. Skoufias), and the National Institutes of Health (grant GM068107 to R.L. Margolis).
Abbreviations used in this paper: APC/C, anaphase-promoting complex/cyclosome; STLC, S-trityl-l-cysteine.
References
- Adams, R.R., S.P. Wheatley, A.M. Gouldsworthy, S.E. Kandels-Lewis, M. Carmena, C. Smythe, D.L. Gerloff, and W.C. Earnshaw. 2000. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr. Biol. 10:1075–1078. [DOI] [PubMed] [Google Scholar]
- Andreassen, P.R., and R.L. Margolis. 1994. Microtubule dependency of p34cdc2 inactivation and mitotic exit in mammalian cells. J. Cell Biol. 127:789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen, P.R., D.K. Palmer, M.H. Wener, and R.L. Margolis. 1991. Telophase disc: a new mammalian mitotic organelle that bisects telophase cells with a possible function in cytokinesis. J. Cell Sci. 99:523–534. [DOI] [PubMed] [Google Scholar]
- Andreassen, P.R., D.A. Skoufias, and R.L. Margolis. 2004. Analysis of the spindle-assembly checkpoint in HeLa cells. Methods Mol. Biol. 281:213–225. [DOI] [PubMed] [Google Scholar]
- Axton, J.M., V. Dombradi, P.T. Cohen, and D.M. Glover. 1990. One of the protein phosphatase 1 isoenzymes in Drosophila is essential for mitosis. Cell. 63:33–46. [DOI] [PubMed] [Google Scholar]
- Bialojan, C., and A. Takai. 1988. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem. J. 256:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito, D.A., and C.L. Rieder. 2006. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr. Biol. 16:1194–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canman, J.C., L.A. Cameron, P.S. Maddox, A. Straight, J.S. Tirnauer, T.J. Mitchison, G. Fang, T.M. Kapoor, and E.D. Salmon. 2003. Determining the position of the cell division plane. Nature. 424:1074–1078. [DOI] [PubMed] [Google Scholar]
- Chen, F., V. Archambault, A. Kar, P. Lio, P.P. D'Avino, R. Sinka, K. Lilley, E.D. Laue, P. Deak, L. Capalbo, and D.M. Glover. 2007. Multiple protein phosphatases are required for mitosis in Drosophila. Curr. Biol. 17:293–303. [DOI] [PubMed] [Google Scholar]
- Cohen, P., C.F. Holmes, and Y. Tsukitani. 1990. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem. Sci. 15:98–102. [DOI] [PubMed] [Google Scholar]
- Crosio, C., G.M. Fimia, R. Loury, M. Kimura, Y. Okano, H. Zhou, S. Sen, C.D. Allis, and P. Sassone-Corsi. 2002. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol. Cell. Biol. 22:874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert, M.S., and J. Thorner. 1989. Putting it on and taking it off: phosphoprotein phosphatase involvement in cell cycle regulation. Cell. 57:891–893. [DOI] [PubMed] [Google Scholar]
- Davis, F.M., T.Y. Tsao, S.K. Fowler, and P.N. Rao. 1983. Monoclonal antibodies to mitotic cells. Proc. Natl. Acad. Sci. USA. 80:2926–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Elzen, N., and J. Pines. 2001. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 153:121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohadwala, M., E.F. da Cruz e Silva, F.L. Hall, R.T. Williams, D.A. Carbonaro-Hall, A.C. Nairn, P. Greengard, and N. Berndt. 1994. Phosphorylation and inactivation of protein phosphatase 1 by cyclin-dependent kinases. Proc. Natl. Acad. Sci. USA. 91:6408–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan, J.H., and N.R. Morris. 1989. The bimG gene of Aspergillus nidulans, required for completion of anaphase, encodes a homolog of mammalian phosphoprotein phosphatase 1. Cell. 57:987–996. [DOI] [PubMed] [Google Scholar]
- Evans, T., E.T. Rosenthal, J. Youngblom, D. Distel, and T. Hunt. 1983. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 33:389–396. [DOI] [PubMed] [Google Scholar]
- Fernandez, A., D.L. Brautigan, and N.J. Lamb. 1992. Protein phosphatase type 1 in mammalian cell mitosis: chromosomal localization and involvement in mitotic exit. J. Cell Biol. 116:1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant, P., and E.A. Nigg. 1992. Cyclin B2 undergoes cell cycle-dependent nuclear translocation and, when expressed as a non-destructible mutant, causes mitotic arrest in HeLa cells. J. Cell Biol. 117:213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geley, S., E. Kramer, C. Gieffers, J. Gannon, J.M. Peters, and T. Hunt. 2001. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol. 153:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace, L., and G. Blobel. 1980. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 19:277–287. [DOI] [PubMed] [Google Scholar]
- Ghiara, J.B., H.E. Richardson, K. Sugimoto, M. Henze, D.J. Lew, C. Wittenberg, and S.I. Reed. 1991. A cyclin B homolog in S. cerevisiae: chronic activation of the Cdc28 protein kinase by cyclin prevents exit from mitosis. Cell. 65:163–174. [DOI] [PubMed] [Google Scholar]
- Giet, R., and D.M. Glover. 2001. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 152:669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer, M., A.W. Murray, and M.W. Kirschner. 1991. Cyclin is degraded by the ubiquitin pathway. Nature. 349:132–138. [DOI] [PubMed] [Google Scholar]
- Guardavaccaro, D., Y. Kudo, J. Boulaire, M. Barchi, L. Busino, M. Donzelli, F. Margottin-Goguet, P.K. Jackson, L. Yamasaki, and M. Pagano. 2003. Control of meiotic and mitotic progression by the F box protein beta-Trcp1 in vivo. Dev. Cell. 4:799–812. [DOI] [PubMed] [Google Scholar]
- Hendzel, M.J., Y. Wei, M.A. Mancini, A. Van Hooser, T. Ranalli, B.R. Brinkley, D.P. Bazett-Jones, and C.D. Allis. 1997. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 106:348–360. [DOI] [PubMed] [Google Scholar]
- Hershko, A., D. Ganoth, J. Pehrson, R.E. Palazzo, and L.H. Cohen. 1991. Methylated ubiquitin inhibits cyclin degradation in clam embryo extracts. J. Biol. Chem. 266:16376–16379. [PubMed] [Google Scholar]
- Holloway, S.L., M. Glotzer, R.W. King, and A.W. Murray. 1993. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell. 73:1393–1402. [DOI] [PubMed] [Google Scholar]
- Hsu, J.Y., Z.W. Sun, X. Li, M. Reuben, K. Tatchell, D.K. Bishop, J.M. Grushcow, C.J. Brame, J.A. Caldwell, D.F. Hunt, et al. 2000. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell. 102:279–291. [DOI] [PubMed] [Google Scholar]
- Ishihara, H., B.L. Martin, D.L. Brautigan, H. Karaki, H. Ozaki, Y. Kato, N. Fusetani, S. Watabe, K. Hashimoto, D. Uemura, et al. 1989. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem. Biophys. Res. Commun. 159:871–877. [DOI] [PubMed] [Google Scholar]
- Ishii, K., K. Kumada, T. Toda, and M. Yanagida. 1996. Requirement for PP1 phosphatase and 20S cyclosome/APC for the onset of anaphase is lessened by the dosage increase of a novel gene sds23+. EMBO J. 15:6629–6640. [PMC free article] [PubMed] [Google Scholar]
- Jiang, W., G. Jimenez, N.J. Wells, T.J. Hope, G.M. Wahl, T. Hunter, and R. Fukunaga. 1998. PRC1: a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol. Cell. 2:877–885. [DOI] [PubMed] [Google Scholar]
- Kops, G.J., B.A. Weaver, and D.W. Cleveland. 2005. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat. Rev. Cancer. 5:773–785. [DOI] [PubMed] [Google Scholar]
- Kwon, Y.G., S.Y. Lee, Y. Choi, P. Greengard, and A.C. Nairn. 1997. Cell cycle-dependent phosphorylation of mammalian protein phosphatase 1 by cdc2 kinase. Proc. Natl. Acad. Sci. USA. 94:2168–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailand, N., C. Lukas, B.K. Kaiser, P.K. Jackson, J. Bartek, and J. Lukas. 2002. Deregulated human Cdc14A phosphatase disrupts centrosome separation and chromosome segregation. Nat. Cell Biol. 4:317–322. [DOI] [PubMed] [Google Scholar]
- Margottin-Goguet, F., J.Y. Hsu, A. Loktev, H.M. Hsieh, J.D. Reimann, and P.K. Jackson. 2003. Prophase destruction of Emi1 by the SCF(betaTrCP/Slimb) ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell. 4:813–826. [DOI] [PubMed] [Google Scholar]
- Martineau, S.N., P.R. Andreassen, and R.L. Margolis. 1995. Delay of HeLa cell cleavage into interphase using dihydrocytochalasin B: retention of a postmitotic spindle and telophase disc correlates with synchronous cleavage recovery. J. Cell Biol. 131:191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull, J., J.J. Blow, and T. Hunt. 1989. Translation of cyclin mRNA is necessary for extracts of activated Xenopus eggs to enter mitosis. Cell. 56:947–956. [DOI] [PubMed] [Google Scholar]
- Mollinari, C., J.P. Kleman, W. Jiang, G. Schoehn, T. Hunter, and R.L. Margolis. 2002. PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J. Cell Biol. 157:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollinari, C., C. Reynaud, S. Martineau-Thuillier, S. Monier, S. Kieffer, J. Garin, P.R. Andreassen, A. Boulet, B. Goud, J.P. Kleman, and R.L. Margolis. 2003. The mammalian passenger protein TD-60 is an RCC1 family member with an essential role in prometaphase to metaphase progression. Dev. Cell. 5:295–307. [DOI] [PubMed] [Google Scholar]
- Murray, A.W. 2004. Recycling the cell cycle: cyclins revisited. Cell. 116:221–234. [DOI] [PubMed] [Google Scholar]
- Murray, A.W., M.J. Solomon, and M.W. Kirschner. 1989. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 339:280–286. [DOI] [PubMed] [Google Scholar]
- Niiya, F., X. Xie, K.S. Lee, H. Inoue, and T. Miki. 2005. Inhibition of cyclin-dependent kinase 1 induces cytokinesis without chromosome segregation in an ECT2 and MgcRacGAP-dependent manner. J. Biol. Chem. 280:36502–36509. [DOI] [PubMed] [Google Scholar]
- Nurse, P. 1990. Universal control mechanism regulating onset of M-phase. Nature. 344:503–508. [DOI] [PubMed] [Google Scholar]
- Oegema, K., M.S. Savoian, T.J. Mitchison, and C.M. Field. 2000. Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis. J. Cell Biol. 150:539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panopoulos, A., M. Harraz, J.F. Engelhardt, and E. Zandi. 2005. Iron-mediated H2O2 production as a mechanism for cell type-specific inhibition of tumor necrosis factor alpha-induced but not interleukin-1beta-induced IkappaB kinase complex/nuclear factor-kappaB activation. J. Biol. Chem. 280:2912–2923. [DOI] [PubMed] [Google Scholar]
- Payton, M., G. Chung, P. Yakowec, A. Wong, D. Powers, L. Xiong, N. Zhang, J. Leal, T.L. Bush, V. Santora, et al. 2006. Discovery and evaluation of dual CDK1 and CDK2 inhibitors. Cancer Res. 66:4299–4308. [DOI] [PubMed] [Google Scholar]
- Peters, J.M. 2002. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell. 9:931–943. [DOI] [PubMed] [Google Scholar]
- Pfleger, C.M., and M.W. Kirschner. 2000. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 14:655–665. [PMC free article] [PubMed] [Google Scholar]
- Potapova, T.A., J.R. Daum, B.D. Pittman, J.R. Hudson, T.N. Jones, D.L. Satinover, P.T. Stukenberg, and G.J. Gorbsky. 2006. The reversibility of mitotic exit in vertebrate cells. Nature. 440:954–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntoni, F., and E. Villa-Moruzzi. 1997. Protein phosphatase-1 alpha, gamma 1, and delta: changes in phosphorylation and activity in mitotic HeLa cells and in cells released from the mitotic block. Arch. Biochem. Biophys. 340:177–184. [DOI] [PubMed] [Google Scholar]
- Queralt, E., C. Lehane, B. Novak, and F. Uhlmann. 2006. Downregulation of PP2A(Cdc55) phosphatase by separase initiates mitotic exit in budding yeast. Cell. 125:719–732. [DOI] [PubMed] [Google Scholar]
- Rape, M., S.K. Reddy, and M.W. Kirschner. 2006. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell. 124:89–103. [DOI] [PubMed] [Google Scholar]
- Skoufias, D.A., C. Mollinari, F.B. Lacroix, and R.L. Margolis. 2000. Human survivin is a kinetochore-associated passenger protein. J. Cell Biol. 151:1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoufias, D.A., S. DeBonis, Y. Saoudi, L. Lebeau, I. Crevel, R. Cross, R.H. Wade, D. Hackney, and F. Kozielski. 2006. S-trityl-L-cysteine is a reversible, tight binding inhibitor of the human kinesin Eg5 that specifically blocks mitotic progression. J. Biol. Chem. 281:17559–17569. [DOI] [PubMed] [Google Scholar]
- Stegmeier, F., and A. Amon. 2004. Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu. Rev. Genet. 38:203–232. [DOI] [PubMed] [Google Scholar]
- Stemmann, O., H. Zou, S.A. Gerber, S.P. Gygi, and M.W. Kirschner. 2001. Dual inhibition of sister chromatid separation at metaphase. Cell. 107:715–726. [DOI] [PubMed] [Google Scholar]
- Stukenberg, P.T., K.D. Lustig, T.J. McGarry, R.W. King, J. Kuang, and M.W. Kirschner. 1997. Systematic identification of mitotic phosphoproteins. Curr. Biol. 7:338–348. [DOI] [PubMed] [Google Scholar]
- Trautmann, S., and D. McCollum. 2002. Cell cycle: new functions for Cdc14 family phosphatases. Curr. Biol. 12:R733–R735. [DOI] [PubMed] [Google Scholar]
- Trinkle-Mulcahy, L., and A.I. Lamond. 2006. Mitotic phosphatases: no longer silent partners. Curr. Opin. Cell Biol. 18:623–631. [DOI] [PubMed] [Google Scholar]
- Vassilev, L.T., C. Tovar, S. Chen, D. Knezevic, X. Zhao, H. Sun, D.C. Heimbrook, and L. Chen. 2006. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Natl. Acad. Sci. USA. 103:10660–10665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Novelle, M.D., V. Esteban, A. Bueno, and M.P. Sacristan. 2005. Functional homology among human and fission yeast Cdc14 phosphatases. J. Biol. Chem. 280:29144–29150. [DOI] [PubMed] [Google Scholar]
- Visintin, R., K. Craig, E.S. Hwang, S. Prinz, M. Tyers, and A. Amon. 1998. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell. 2:709–718. [DOI] [PubMed] [Google Scholar]
- Wera, S., and B.A. Hemmings. 1995. Serine/threonine protein phosphatases. Biochem. J. 311:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley, S.P., E.H. Hinchcliffe, M. Glotzer, A.A. Hyman, G. Sluder, and Y. Wang. 1997. CDK1 inactivation regulates anaphase spindle dynamics and cytokinesis in vivo. J. Cell Biol. 138:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieve, G.W., D. Turnbull, J.M. Mullins, and J.R. McIntosh. 1980. Production of large numbers of mitotic mammalian cells by use of the reversible microtubule inhibitor nocodazole. Nocodazole accumulated mitotic cells. Exp. Cell Res. 126:397–405. [DOI] [PubMed] [Google Scholar]
- Zou, H., T.J. McGarry, T. Bernal, and M.W. Kirschner. 1999. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 285:418–422. [DOI] [PubMed] [Google Scholar]
- Zur, A., and M. Brandeis. 2001. Securin degradation is mediated by fzy and fzr, and is required for complete chromatid separation but not for cytokinesis. EMBO J. 20:792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]