Abstract
The volatility of alcohol promotes the movement of alcohol from the bronchial circulation across the airway epithelium and into the conducting airways of the lung. The exposure of the airways through this route likely accounts for many of the biologic effects of alcohol on lung airway functions. The impact of alcohol on lung airway functions is dependent on the concentration, duration and route of exposure. Brief exposure to mild concentrations of alcohol may enhance mucociliary clearance, stimulates bronchodilation and probably attenuates the airway inflammation and injury observed in asthma and COPD. Prolonged and heavy exposure to alcohol impairs mucociliary clearance, may complicate asthma management and likely worsens outcomes including lung function and mortality in COPD patients. Non-alcohol congeners and alcohol metabolites act as triggers for airway disease exacerbations especially in atopic asthmatics and in Asian populations who have a reduced capacity to metabolize alcohol. Research focused on the mechanisms of alcohol-mediated changes in airway functions has identified specific mechanisms that mediate alcohol effects within the lung airways. These include prominent roles for the second messengers calcium and nitric oxide, regulatory kinases including PKG and PKA, alcohol and acetaldehyde-metabolizing enzymes such as aldehyde dehydrogenase type 2 (ALDH2). The role alcohol may play in the pathobiology of airway mucus, bronchial blood flow, airway smooth muscle regulation and the interaction with other airway exposure agents, such as cigarette smoke, represent opportunities for future investigation.
Keywords: cilia, mucociliary clearance, cigarette smoke, alcohol, ethanol, cAMP, PKA, PKG, lung epithelium, COPD, Chronic Obstructive Pulmonary Disease, acetaldehyde dehydrogenase type, ALDH2
Introduction
The exchange of gases between the outside environment and the bloodstream is the primary function of the lung. This requires the bidirectional movement of air through the conducting airways to alveoli where fresh air is exposed to capillary blood from the pulmonary circulation. Matching airflow with blood flow is critical for normal gas exchange and requires a delicate balance between the blood and air distribution systems.
The conducting airways of the lung, including the trachea, bronchi and bronchioles, function to distribute air throughout the lung and represent the proximal and often rate-limiting component of the air distribution system. Normal lung airways branch and taper from the trachea down to terminal bronchi providing balanced and regulable airflow throughout the lung. By virtue of their proximal location in lung airflow distribution, the conducting airways are the first interface of the lung with the outside environment. Despite this front line position, the airways below the vocal cords are normally sterile because of highly effective defense mechanisms (Laurenzi et al., 1961). It is not surprising, however, that lung airways are at great risk for injury and infection from the outside environment. One well recognized risk factor for developing lung infections is heavy alcohol intake.
Heavy alcohol intake has been known for centuries to impair lung defenses. Indeed, the alcoholic with pneumonia as the prototype of the immunocompromized host is well known to every first year medical student (Chomet and Gach, 1967). Our understanding of how alcohol impairs lung defenses continues to grow. An ever-expanding body of evidence points to multiple immune mechanisms by which alcohol intake compromises lung defenses and has been previously reviewed (Bomalaski and Phair, 1982; Happel and Nelson, 2005). While innate and acquired lung immune mechanisms are vitally important, the effects of alcohol intake on the functions of lung airways are poorly understood. Importantly and perhaps not as well known, alcohol intake is also clearly linked to a variety of airway diseases likely playing pathogenic, treatment and protective roles.
Diseases of the conducting airways are extremely common with prominent examples including bronchitis, asthma and chronic obstructive pulmonary disease (COPD). Although it is not widely recognized by many clinicians, alcohol has long been considered both a treatment and a cause for a variety of airway diseases. This review focuses on our current understanding of alcohol’s impact on airway functions based on clinical and experimental research. What emerges is that alcohol has a considerable and largely unrecognized influence on airway function in health and disease. Much of this impact stems from the unique vapor characteristics of alcohol and its interplay with the bronchial circulation.
Alcohol Vapor Characteristics in the Airways
Clinicians and physiologists commonly believe that the alcohol present in exhaled air during alcohol consumption comes from alcohol that is vaporized from the alveolar-capillary interface of the pulmonary circulation. Surprisingly, this is not the case. Careful studies by George and colleagues show that almost all of the exhaled alcohol is derived from the bronchial and not the pulmonary circulation (George et al., 1996). The unique volatility characteristics of alcohol are informative. During alcohol ingestion, alcohol freely diffuses from the bronchial circulation directly through the ciliated epithelium where it vaporizes as it moves into the conducting airways (George et al., 1996). Indeed, alcohol vapor excreted into the airways in this manner forms the basis of the breath test used to estimate blood alcohol levels (Hlastala, 1998). Moreover, vaporized alcohol can deposit back into the airway lining fluid to be released again into the airways during exhalation. This “recycling” of alcohol vapor results in repeated exposure of the airway epithelium to high local concentrations of alcohol (George et al., 1996). In this manner, the epithelium of the conducting airways is continually exposed to ethanol during alcohol ingestion.
Circumstantial evidence also exists that implicates the importance of the airways in alcohol excretion. This comes from clinical studies of the utility of estimating blood alcohol concentration (BAC) with the breath test (Breathalyzer) in patients with chronic obstructive pulmonary disease (COPD). A study of ten older male patients with COPD, given a standard alcohol drink, found that exhaled Breathalyzer alcohol levels in the COPD patients did not correlate with BACs compared to the linear relationship of Breathalyzer levels with BACs in normal subjects (Russell and Jones, 1983). A second study showed that Breathalyzer levels significantly underestimated BACs in patients with COPD as a function of age (Wilson et al., 1987). These findings were confirmed in a third study that demonstrated poor correlation between exhaled alcohol concentrations and BACs in patients with COPD and asthma (Honeybourne et al., 2000). These studies in patients with airway disease corroborate the importance of the airways in alcohol excretion. When the volatility of alcohol and the role the bronchial circulation plays in alcohol excretion are considered, it is not surprising that alcohol alters critical airway functions like mucociliary clearance.
Alcohol and Mucociliary Clearance
The mucociliary apparatus consists of mucus secreting cells, sero-mucinous bronchial glands and ciliated cells that line the conducting airways from the trachea to the terminal bronchi deep in the lung. This system traps inhaled particles and debris in secreted mucus, which is then propelled up and out of the lung via the escalator-like function of the waves created by beating cilia. Normal mucociliary clearance ensures a sterile environment in the lung below the vocal cords (Laurenzi et al., 1965; Laurenzi et al., 1963; Laurenzi et al., 1961). This is critical for airflow and gas exchange, and prevents the inhalation of airborne infectious particles laden with bacteria, fungi and viruses. Failure of this system results in recurrent bronchitis, pneumonia and airway deformity in the form of bronchiectasis (Noone et al., 2004). A growing body of evidence points to alcohol as an important modifier of mucociliary clearance, which is the first line of defense for the lungs.
Clinical Studies of Alcohol and Mucociliary Clearance
Although clinicians have long suspected that mucociliary clearance is reduced in heavy drinkers (Heinemann, 1977), few clinical studies have directly examined the effect of alcohol ingestion of mucociliary clearance. Venizelos measured radiolabelled particle clearance in 12 normal volunteers following ingestion of a standard alcohol drink (0.5 g alcohol/kg in juice) or juice alone (Venizelos et al., 1981). As a group there was no difference between particle clearance rates following alcohol or juice alone but the variance of clearance time was greater following alcohol ingestion and was related to each subject’s previous alcohol intake history. In subjects with a “moderate” history of drinking, defined as at least one drink per week but less than two drinks per day, clearance was notably faster following alcohol ingestion. In contrast, half of the subjects with a history of “mild” alcohol ingestion, defined as less than one drink per week and no more than two drinks on one occasion, clearance was significantly slowed by alcohol. This variance could not be explained by other obvious factors such as cigarette smoking. Another study examined ciliary beat frequency (CBF) from airway tissue obtained during bronchoscopy under general anesthesia from 50 subjects with respiratory problems in which alcohol intake ranged from “none” to “heavy” (Dulfano et al., 1981). They found no differences in CBF among the subjects related to alcohol intake. It is difficult to draw firm conclusions about the effects of alcohol on mucociliary function from these studies since alcohol intake, including recent drinking, were not carefully assessed and artifacts from ex situ cilia analysis do not likely represent the in vivo situation. In contrast to these few clinical studies, a larger body of literature indicates both short and long term effects of alcohol on the mucociliary apparatus.
Basic Science Studies of Alcohol and Mucociliary Clearance
Early basic studies of alcohol on airway cilia could not quantify CBF and instead measured the time to complete cessation of ciliary motion (ciliostasis) following direct application of alcohol to airway tissues. These authors determined that very high concentrations of alcohol (4–10% or 0.8–3.2 M) caused concentration-dependent ciliostasis (Nungester and Klepser, 1938; Purkinje and Valentine, 1835) while lower concentrations (1%) did not (Dalhamn et al., 1967). While informative, ciliostasis is not a very physiologic endpoint and the extremely high and biologically irrelevant concentrations of alcohol used in these early studies limit their applicability.
Later more elegant in vivo studies in mice and kittens by Laurenzi demonstrated profound effects caused by injections of intraperitoneal (IP) alcohol on mucociliary clearance (Laurenzi and Guarneri, 1966). IP alcohol, at 5–21% concentrations that induced coma, caused concentration- and time-dependent slowing of clearance of inhaled staphylococci in mice. At the highest concentration of IP alcohol used (21%) clearance was slowed five-fold compared to control mice and there was a strong direct correlation between the reductions of airway clearance with the blood alcohol concentrations. Importantly, in the same study the investigators directly observed tracheal clearance of inert carbon particles following IP alcohol injection of anesthetized kittens. Alcohol caused a rapid and reversible concentration-dependent slowing of airway particle clearance compared to control kittens. This important study established that alcohol clearly impairs mucociliary clearance. While the focus of these experiments was mucociliary clearance, the impact of alcohol on mucus production was not examined.
The impact of alcohol on airway mucus represents an understudied area. Only a few studies have examined the effects of alcohol on airway mucus. Boyd reported that inhaled alcohol, in a dose-dependent manner, augmented the volume and mucus content from the lungs of anesthetized rabbits at very high doses (5 ml/kg) of inhaled alcohol (Boyd and Sheppard, 1969). Using the frog palate model, Leitch found that high concentrations of alcohol (3–5% or 0.6–1.1 M) depressed both mucus clearance and secretion (Leitch et al., 1985). The applicability of the frog palate as a model of human airways is uncertain and the extremely high concentrations of alcohol used in these experiments are not relevant to human alcohol consumption. Another study in cultured human bronchial epithelial cells found that alcohol caused a concentration- and time-dependent increase in the expression of the tracheo-bronchial mucin (TBM) gene (Verma and Davidson, 1997). An eight-fold change in TBM expression was simulated by 100 mM alcohol. This finding suggests that alcohol regulates mucin expression in the airway epithelium at a biologically relevant concentration.
The first careful in vitro experiments that examined the effects of modest concentrations of alcohol on CBF in tracheal tissues were done in airway tissue from unanaesthetized sheep during fiberoptic bronchoscopy (Maurer and Liebman, 1988). These investigators found that CBF was stimulated by low concentrations of alcohol (0.01–0.1% or ≈ 2–20 mM), not changed by modest concentrations of alcohol (0.5–1.0% or ≈ 100–200 mM) and slowed at higher concentrations of alcohol (2% or ≈ 400 mM). Subsequent studies in bovine tracheal ciliated cells established that alcohol stimulation of CBF was dependent on nitric oxide production in airway epithelium (Sisson, 1995) and required the dual activation of both cyclic AMP- and cyclic GMP-dependent protein kinases, PKA and PKG, in ciliated cells (Sisson et al., 1999; Wyatt et al., 2003). This transient alcohol stimulation effect on cilia was recapitulated in vivo in alcohol-fed rats (Wyatt et al., 2004). In this model, 1 week of feeding 36% alcohol increased baseline CBF 40% over control animals and was comparable to stimulation with an exogenous beta agonist. These findings indicate that brief exposure to alcohol stimulated ciliary motility both in vitro and in vivo.
The stimulation of ciliary motility by biologically relevant concentrations of alcohol was surprising since higher ciliary motility should enhance mucociliary clearance and did not fit with the conventional wisdom that lung clearance is impaired in heavy drinkers. Some resolution of this apparent paradox came from subsequent experiments that demonstrated that alcohol-induced CBF stimulation in vitro was transient and quickly followed by both the induction of an alcohol-sensitive phosphodiesterase (Forget et al., 2003) and downregulation of PKA in ciliated cells exposed to alcohol for more than 6 hours (Wyatt and Sisson, 2001). The consequence of prolonged exposure to alcohol was desensitization of the mucociliary apparatus, meaning that cilia could no longer be stimulated during stress, such as following aspiration of bacteria. This hypothesis better fit the notion that airway mucociliary clearance is impaired in chronic drinkers. Whether these mechanisms were operative in vivo required studies of cilia in animals.
Studies of mucociliary function in animals drinking alcohol have provided important information about both the impact and the mechanism of alcohol-impaired airway clearance in vivo. Rats fed alcohol for six weeks demonstrated slowed cilia beating and desensitization of airway PKA activity (Wyatt et al., 2004). Importantly, bacterial clearance was impaired by alcohol feeding in this same model and the degree of impaired clearance correlated with the degree of cilia desensitization (Vander Top et al., 2005). This same finding was reproduced in mice ingesting alcohol in their drinking water (Elliott et al., 2007). Taken together, these studies fully recapitulated the in vitro findings of alcohol-desensitization of ciliary kinases. At this juncture, alcohol downregulation of airway ciliary PKA represents the most likely mechanism that causes alcohol-induced impairment of mucociliary clearance.
Summary of Alcohol and Mucociliary Clearance
Alcohol alters airway mucociliary clearance, which is dependent upon the dose and duration of alcohol exposure. There is a notable scarcity of studies of the effect of alcohol on airway mucus. Most studies have focused on the effect of alcohol on clearance and ciliary motility. Brief exposure to modest doses of alcohol stimulates ciliary motility through the production of nitric oxide and the dual activation of the cyclic nucleotide-dependent kinases, PKG and PKA. Although one can hypothesize that brief exposure to modest amounts of alcohol improves airway clearance, there are no studies to directly support this hypothesis. In contrast, prolonged exposure to high concentrations of alcohol desensitizes airway cilia to external stimuli and impairs airway clearance of bacterial pathogens. In heavy drinkers, alcohol-induced impairment of mucociliary clearance represents a major breach of lung host defenses and contributes to the high incidence of lung infections encountered in heavy drinkers.
Alcohol and Asthma
Asthma, defined as reversible airflow obstruction, has been linked to alcohol intake for millennia. Like so many complex associations with alcohol use, alcohol has been suggested to be both a trigger of asthma and a treatment for asthma.
Clinical Studies of Alcohol and Asthma
Alcohol has been used as a treatment for asthma since antiquity. The earliest indication of alcohol as a treatment for asthma appears on Egyptian papyri ca. 2000 B.C (Leake, 1952). The term asthma likely encompassed any number of chest ailments in ancient Egypt where beer and wine were prescribed for chest tightness with apparent relief of asthma symptoms (Ayres, 1987). In ancient Greece Hippocrates popularized alcohol as treatment for a variety of ailments and suggested that wine reduces sputum production, a problem that plagues asthmatics having exacerbations (Lucia, 1963). Since ancient times, the use of alcohol for the treatment of asthma is anecdotal until the last two centuries where accounts are more detailed.
In the 19th century, Hyde Salter reported self-administration of high amounts of oral alcohol by three of his patients with severe asthma exacerbations and noted improvement of their symptoms (Salter, 1863). Soon after this finding was published, intermittent reports on the use of oral administration of pure alcohol diluted in water for treatment of asthma appear (Leffman, 1885; Richardson, 1881). Indeed, the use of alcohol as a treatment was widespread by physicians in the United States well into the early 20th century until Prohibition when its use was officially renounced by the American Medical Association (AMA, 1922). Following the repeal of Prohibition in 1933, more rigorous studies using alcohol as a treatment for asthma began to appear.
In 1963 Herxheimer measured lung vital capacity (VC) in normal subjects and asthmatics following the ingestion of brandy, vodka or pure ethanol (Herxheimer and Stresemann, 1963). Alcohol ingestion did not change VC in normal subjects but increased VC between 6–38% in most of the asthmatics and was accompanied by subjective improvement in their asthma symptoms. The VC improvement began about 10 minutes after alcohol ingestion, peaked by 30 minutes and returned to baseline by two hours. The authors concluded that alcohol had a clear anti-asthmatic effect confirming the findings of Salter from a century before.
Soon thereafter, a small but important clinical study by Ayres examined the effects of drinking alcohol in asthma. Changes in airflow were measured following the ingestion of different concentrations of pure ethanol (diluted in water) in 5 normal subjects and 5 patients with asthma (Ayres et al., 1982). Two of the normal subjects and 3 of the asthmatic patients had a slight decrease in specific airways conductance with 20% alcohol within 5 minutes of quickly swallowing the whole drink. Higher concentrations of alcohol (60%), when sipped slowly over 5 minutes, resulted in significant increases in airway conductance in 4 of 5 of the asthmatics. This study suggests that while alcohol can immediately trigger an initial small upper airway irritant response, a separate slow bronchodilator effect can be observed in asthmatics.
The first reported use of intravenous (IV) alcohol for the treatment of asthma appeared in 1947 when Brown infused 5% ethanol into children with severe asthma attacks who were unresponsive to conventional asthma therapy (Brown, 1947). Five of the six patients improved with the alcohol infusion and no adverse reactions were reported. This report suggested that pure alcohol, when administered intravenously and, in the absence of any other ingredients, acted as a bronchodilator and could be used as a treatment of asthma. A later report noted that asthmatics cleared intravenous alcohol from the bloodstream significantly faster than controls (Sotaniemi et al., 1972) and was confirmed by a subsequent report (Korri and Salaspuro, 1988). They speculated that the difference in alcohol clearance was likely related to concomitant medication use or hypoxia and hypercapnea which can cause micosomal enzyme induction in the liver of the asthmatic patients that increased alcohol metabolism. The most recent study of intravenous ethanol on airflow examined the changes in spirometry, lung volumes and airway conductance that followed infusion of three different concentrations of ethanol (2%, 4% and 8% v/v in saline) in 5 normal subjects and 5 atopic asthmatics (Ayres and Clark, 1983b). While no change in any pulmonary function was noted in the normal subjects at any concentration of IV alcohol, concentration-dependent bronchodilation occurred in all of the asthmatics. At the highest concentration (8%) IV alcohol caused a 33% increase in airway conductance in the asthmatics, which was roughly one third of the response that inhaled salmeterol, a beta-agonist, could induce in the same patients. No side effects were reported in either test group. While this study was small, it demonstrated the modest bronchodilator properties of IV ethanol.
The implication that a pure alcohol infusion acted as a bronchodilator and did not worsen asthma was important since some atopic patients report bronchospasm following ingestion of alcoholic beverages. This point was made in a small but elegant study by Breslin in 1973 of eleven subjects with asthma who reported worsening of their asthma symptoms following the ingestion of an alcoholic beverage (Breslin et al., 1973). The authors were able to provoke bronchospasm in the laboratory in six of the eleven subjects challenged with the offending alcoholic beverage precipitating a ≥ 15% reduction in the forced expiratory flow in the first second (FEV1) on spirometry. Importantly, in three of these patients, drink-precipitated bronchospasm was not triggered by an oral ingestion of an equivalent amount of pure alcohol in water implicating the non-alcohol components of the beverage as the likely asthma trigger. Indeed, treatment with disodium cromoglycate, a drug that inhibits mast cell granule release and used in the treatment of asthma, prevented bronchospasm to the offending alcoholic beverage. Similar findings were obtained in another study that implicated the sulfur dioxide content in red wine as a likely trigger for bronchospasm in asthmatics rather than the alcohol itself (Dahl et al., 1986). These studies indicate that both the purity (pure ethanol vs. an alcoholic beverage) and the route (oral vs. intravenous) are factors that may determine how alcohol might modify airway function. An excellent review of alcoholic drinks as triggers for asthma has been previously published (Vally et al., 2000).
A third route of alcohol exposure, unique to the lung, is by inhalation. Little is known about the effect of inhaled alcohol on the airways. Zuskin exposed healthy volunteers to a nebulized solution of 25% alcohol in water and measured flow rates and spirometry (Zuskin et al., 1981). Compared to nebulized saline, nebulized alcohol triggered coughing and caused a small but significant reduction in airflow that persisted for 90 minutes in all subjects, consistent with an irritant effect. This was anecdotally confirmed in case reports of two mild asthmatics who developed bronchospasm following exposure to 20% aerosolized ethanol alone as part of a drug safety protocol (Hooper et al., 1995). These authors concluded that the use of ethanol as a carrier for inhaled drug formulations is unpredictable and potentially hazardous in asthmatics (Hooper et al., 1995). This is also potentially important because of the rapidly increasing production and use of ethanol as a fuel additive. Consumers of such fuels or workers involved with their production will likely be exposed to ethanol vapors often combined with other vapors (Chu et al., 2005). Another alcohol vapor exposure is in the form abusing “alcohol-with-out-liquid” (AWOL). With AWOL alcohol is aerosolized through a nebulizer and has become fashionable in Europe and Asia as way to become intoxicated without the side effects of drinking (Press, 2004). The increase in the use of ethanol-supplemented fuels and the abuse potential of AWOL will likely stimulate more research in this interesting area. At this point it is safe to say that our knowledge about the influence of inhaled alcohol on airway function is unsatisfactory. This is in contrast to our knowledge of alcohol intake and asthma from population studies.
The first population survey to assess the potential impact of alcohol on asthma was reported by Ayres and Clark in 1983 (Ayres and Clark, 1983a). Using a questionnaire, they queried 168 patients with known asthma from a chest clinic at four hospitals excluding asthmatics with cardiac disease, other pulmonary diseases and lifetime non-drinkers. The questions focused on the severity, duration and variation of their asthma, their smoking history, their pattern and degree of alcohol consumption and their association of drinking with their asthma symptoms. One third of respondents reported wheezing with a particular form of alcohol while one sixth of respondents reported wheezing with more than one type of alcohol beverage. Wine was the most likely alcoholic beverage to trigger wheezing (30%) with beer and whiskey triggering wheezing less often in 23% and 16% of asthmatics, respectively. Conversely, 23% of their respondents reported that alcoholic drinks improved their asthma, especially exacerbations. Of these 39 patients who reported improvement of their asthma symptoms, 29 thought that alcohol promoted relaxation, 21 thought alcohol reduced wheezing and 15 reported that alcohol helped loosen up their airway secretions. Interestingly, 14 patients stated that one form of alcohol triggered wheezing while another form improved their asthma symptoms. Limitations of this study were the high fraction of atopic asthmatics (84%), the exclusion of mild asthmatics, the high proportions of males (63%) and the inability to determine the prevalence of alcohol consumption among asthmatics. These findings were validated in a larger study that also identified sulfite additives and even salicylates in wines as triggers for asthma (Vally et al., 2000). The important findings from these studies are that: 1) the non-alcohol congeners present in different alcoholic beverages may act as triggers for asthma; and 2) alcohol may, in some asthmatics, act as a bronchodilator. Other than congeners, the agent most commonly associated with drinking that clearly triggers bronchospasm is the product of ethanol oxidation, acetaldehyde.
Acetaldehyde is produced by the metabolism of ethanol through the action of alcohol dehydrogenases. Acetaldehyde has long been recognized as a trigger for asthma in Asians and is referred to as “alcohol-induced bronchial asthma” (Shimoda et al., 1996). The most susceptible individuals are Asians who have greatly reduced function of the enzyme aldehyde dehydrogenase isoform 2 (ALDH 2) and can be identified through genetic testing and/or ethanol challenge testing (Matsuse et al., 2001). About half of Japanese have inadequate ALDH2 activity and cannot effectively metabolize acetaldehyde. This results in facial flushing, wheezing and other undesirable side effects following the ingestion of modest amounts of alcohol (Gong et al., 1981). Bronchospasm following alcohol ingestion is well described in asthmatics of Japanese descent (Watanabe, 1991) and is closely linked to the ALDH2 genotype (Shimoda et al., 1996).
The mechanism for acetaldehyde-triggered bronchospasm in these individuals is thought to occur through a non-cholinergic pathway (Geppert and Boushey, 1978) related to degranulation of mast cells triggered by high blood acetaldehyde levels that result in the release of histamine and other bronchoconstricting mediators (Shimoda et al., 1996). Interestingly, Myou found that inhaled ethanol did not trigger bronchospasm in Japanese subjects with alcohol-induced asthma. Indeed, inhaled ethanol attenuated methacholine-induced bronchospasm in these asthmatics (Myou et al., 1996). This is likely due to the inability of the airway epithelium to significantly metabolize ethanol into acetaldehyde. This study is consistent with the hypothesis that alcohol, in the absence of acetaldehyde or congeners, does not trigger asthma even in susceptible individuals with impaired ALDH2 function. This hypothesis is further supported by an animal study that determined that aerosolized acetaldehyde but not ethanol induced histamine-mediated bronchoconstriction in guinea pigs (Myou et al., 1994).
In summary, alcohol by itself is a modest bronchodilator and likely relaxes bronchial smooth muscle. The route, duration and concentration of alcohol intake are variables that likely modify this response. Bronchoconstriction and wheezing following ingestion of alcoholic beverages is most likely related to non-alcohol congeners present in the beverages or the production of high concentrations of acetaldehyde in susceptible individuals with the low functioning ALDH2 genotype.
Basic Science Studies of Alcohol and Asthma
Classic animal studies of asthma have utilized intact animal sensitization with ovalbumin followed by re-exposure to ovalbumin to explore the mechanisms of antigen-induced bronchospasm. Fleisch and colleagues, while studying bronchial contraction of airways from the excised lungs of ovalbumin-sensitized guinea pigs, observed that when alcohol or propylene glycol were used to dissolve some of their reagents, ovalbumin- but not prostaglandin F2α-triggered bronchoconstriction was blocked (Fleisch et al., 1976). Experiments with two other alcohols, propanol and butanol, similarly blocked ovalbumin-triggered bronchoconstriction. The concentration of ethanol used in these experiments was quite high (1.5% or 310 mM), which mimics those commonly used to dissolve reagents into buffers for such experiments. Fleisch’s findings extended an earlier report that very high concentrations of ethanol (2.4 % or 500 mM) inhibited antigen-induced histamine release from guinea pig lung tissue (Mongar and Schild, 1957). To view these concentrations of alcohol in a clinical context, a 1.5% solution of ethanol is approximately 17 times higher than the blood concentration that is used in most areas to define legal intoxication (0.08% or 18 mM). However, alcohol levels of 200–300 mM are rare but have been recorded in heavily intoxicated individuals treated in emergency departments. Two important findings emerged from these early studies: 1) high concentrations of alcohols modify the airway responses to bronchoprovocants; and 2) the use of alcohols as vehicles to dissolve reagents can obscure the findings of bronchial reactivity. These findings suggest that alcohol can relax constricted airway smooth muscle, which is a significant factor in the pathogenesis of asthma.
The potential mechanism(s) of alcohol-induced bronchial relaxation have been explored in a few studies. Extrapolations from studies that examine the effects of alcohol on skeletal and cardiac myocytes provide clues as to how alcohol might relax airway smooth muscle. Studies of canine cardiac muscle demonstrate that very high concentrations of ethanol (> 500 mM) block calcium uptake and binding of calcium to cardiac microsomes (Swartz et al., 1974). A study by Puszkin in 1975 demonstrated that ethanol and its metabolite, acetaldehyde, are capable of reversibly inhibiting adenosine monophosphate-(ADP) induced re-association of skeletal muscle cell actin and myosin (Puszkin and Rubin, 1975). While these studies proposed that such mechanisms might drive alcohol-associated cardiomyopathy, it is also conceivable that it could promote relaxation of bronchial smooth muscle.
Richards determined that modest and biologically relevant concentrations of alcohol (0.13%–0.16% or 8–34 mM) caused concentration-dependent hyperpolarization and suppression of membrane action potentials in canine tracheal smooth muscle preparations (Richards et al., 1989). This effect was blocked by a β-adrenergic blocker and was not reproduced in isolated first passage cultured airway epithelial cells. These findings suggested that autonomic innervation and functional β-adrenergic receptors participate in alcohol-induced relaxation of airway smooth muscles. The applicability of this study, however, is uncertain since most of the bronchoreactivity of asthma occurs in the small airways and not the trachea. Furthermore, the role of adrenergic innervation, while important in the canine airway, is minor in the regulation of human airways.
Direct effects of alcohol on airway smooth muscle function have been suggested by some studies. A study of isolated guinea pig tracheal smooth muscle tone demonstrated that alcohol causes concentration-dependent contraction of airway smooth muscle (Jakupi et al., 1986). This effect was partially reduced by histamine or the alpha-adrenergic blockade, but completed abolished by calcium channel blockade, suggesting a calcium flux mediated alcohol-triggered airway smooth muscle contraction in this model. The high concentrations of alcohol used in this study undermine the applicability of these findings. In contrast, a more recent study in permeabolized canine smooth airway cells by Hanazaki and colleagues found that alcohol promotes concentration-dependent airway smooth muscle relaxation and increased sensitivity to calcium, independent of regulatory myosin light chain phosphorylation (Hanazaki et al., 2001). This study suggests a direct effect of alcohol on calcium-regulated smooth muscle tone and is consistent with the observation that alcohol is a bronchodilator.
Another mechanism that might explain alcohol-mediated bronchodilation is through release of nitric oxide (NO). Alcohol rapidly stimulates the production of NO from cultured bronchial epithelial cells (Wyatt et al., 2003) through the activation of a constitutive nitric oxide synthase (NOS), mostly likely the endothelial NOS isoform (eNOS or NOS-3). NO can act as a weak bronchodilator in asthmatics but not in normal subjects (Högman et al., 1993). These findings, while consistent with a bronchodilator action of alcohol, are difficult to reconcile with studies that measure exhaled NO following alcohol intake. Persson demonstrated that IV ethanol produced concentration-dependent reduction of exhaled NO in anesthetized rabbits (Persson and Gustafsson, 1992). Since almost all exhaled NO is derived from the conducting airways and not the alveolar space, the authors speculated that the alcohol-mediated decrease in NO was likely linked to airway function. This speculation was further substantiated by a study of exhaled NO in normal and asthmatic subjects by Yates who found that oral ingestion of a modest amount of alcohol was associated with a significant reduction of exhaled NO in asthmatic but not normal subjects (Yates et al., 1996). They hypothesized that this was due to inhibition of the inducible isoform of nitric oxide synthase (iNOS or NOS-2), which is high in asthmatics, linked to airway inflammation and is not elevated in normal subjects. Taken together, there are intriguing links between alcohol’s modulation of airway NO and changes in airflow although this association is poorly understood.
Lastly, there are animal data suggesting that alcohol can promote neurogenic-driven airway inflammation. Trevisani and colleagues demonstrated in guinea pigs that alcohol intake triggers airway inflammation via a transient receptor potential vanilloid-1 (TRPV1) resulting in a calcium-dependent release of neuropeptides that contracted airway smooth muscle (Trevisani et al., 2004). The authors suggested that neurogenic airway inflammation may be an important mechanism by which alcohol causes asthma, which might be treatable with inhaled steroids (Antonicelli et al., 2006b). There is even an anecdotal report that suggests small amounts of alcohol present in some inhaled corticosteroid preparations can act as a trigger for asthma for certain individuals (Antonicelli et al., 2006a).
Summary of Alcohol and Asthma
Pure ethanol is a moderately effective and transient bronchodilator and likely relaxes airway smooth muscle tone. The mechanisms responsible for alcohol-induced relaxation of airways are poorly understood and may include receptor-and non receptor-mediated signal transduction pathways involving calcium and/or nitric oxide as second messengers. Many non-alcohol components of alcoholic beverages likely act as triggers for asthma in sensitized individuals and as such are not different from other asthma triggers. Acetaldehyde, the primary metabolite of ethanol, can trigger bronchoconstriction in asthmatics with genetically reduced ALDH2 activity and represents a significant trigger for asthma in certain Asian populations.
Alcohol and Chronic Obstructive Pulmonary Disease (COPD)
When airflow obstruction is not reversible, it is called chronic obstructive pulmonary disease (COPD). Individuals with COPD typically have, to varying degrees, elements of asthma, bronchitis and emphysema. The presence of obstruction on lung airflow and volume measurements (spirometry) almost always indicates airways disease within the lung.
Airflow obstruction diseases continue to increase in prevalence and that chronic obstructive pulmonary disease (COPD) will become the third most common cause of death in the United States by the year 2020 (Mannino et al., 2003). Aside from smoking, which is a well-known risk factor for developing COPD, little is known about other factors that impact risk for developing airflow obstruction. The term “whiskey bronchitis” is an expression that was often used to describe the high prevalence of bronchitis in alcoholics (Lyons et al., 1986). Such common clinical observations likely prompted George Burch to write a provocative editorial in 1967 in the American Heart Journal entitled “Alcoholic lung disease-An hypothesis” (Burch and DePasquale, 1967). In this editorial he made a cogent case for the lung being a prime candidate for alcohol-induced tissue injury. He asserted that this is due to the lung’s delicate structure and its exposure to the entire cardiac output containing alcohol that has escaped first pass metabolism in the liver. Although we have not yet conclusively proven Burch’s hypothesis, there is growing evidence that alcohol plays a role in the pathogenesis of COPD.
Clinical Studies of Alcohol and COPD
Unlike studies that have linked asthma with alcohol for millennia, the associations of alcohol intake with COPD are relatively new. There are several likely reasons for this: 1) chronic bronchitis and emphysema were not recognized as health care problems separate from asthma until early in the 19th century (Badham, 1814); 2) the primary method used to measure airflow obstruction and recognize COPD, spirometry, was not available until the mid-19th century (Hutchinson, 1846); 3) tobacco use was not widespread in the general population until the 20th century; 4) definitions of COPD were not formulated until the mid-20th century (Petty, 2002); 5) in contrast to COPD, which is a slow and largely irreversible process, the reversibility of asthma makes it easier to study in a laboratory setting; and most importantly 6) the prevalence rates of smoking and drinking are so high it is difficult to separately determine their impact on the development of COPD. For these reasons only a modest body of recent literature exists espousing an association between COPD and alcohol intake.
A survey by Saric of factory workers found that heavy alcohol intake of wine and spirits was associated with sputum production, bronchitis, wheezing, and airflow obstruction as measured by spirometry (Saric et al., 1977). While this study lacked precise definitions of smoke and alcohol exposure, the association between COPD findings and alcohol intake persisted in the group of non-smokers implying that smoking alone could not explain the findings. Furthermore, combined exposure to smoke and alcohol was greater than either exposure alone suggesting a synergism between smoke and alcohol exposure and COPD. The authors recommended that alcohol consumption should be taken into consideration in any evaluation of the prevalence, incidence and etiology of the disease.
Unlike asthma studies, where spirometry can be measured before and after alcohol ingestion, lung function studies in COPD patients ingesting alcohol are challenged by trying to measure an acute change in a disease that is by definition chronic. This was aptly demonstrated in a small study of patients with severe bronchitis who, when given a standard alcohol drink, demonstrated no change in airflow obstruction and arterial blood gas measurements (Sovijarvi et al., 1978). Another approach is to link COPD severity to alcohol consumption. This approach was taken in an innovative analysis of drinking patterns among severe COPD patients (Jalleh et al., 1993). These investigators found that among the 73 patients with COPD, right-sided heart failure was significantly associated with high alcohol intake. This syndrome, known as cor pulmonale, occurs following sustained increases in pulmonary artery pressure caused by chronic lung diseases. As these two studies illustrate, our ability to study alcohol intake on the pathophysiology of COPD in the laboratory is limited. As a consequence, most of our knowledge of the associations of alcohol with COPD is derived from epidemiologic studies.
Many studies have assessed pulmonary function in alcoholics. While alcoholics represent a minority of the drinking public, these results are informative. Banner observed that nearly half of the patients admitted to an alcohol detoxification unit had airflow obstruction on spirometry and almost all had in gas diffusion impairment that could not be explained on the basis of cigarette smoking (Banner, 1973). The findings were confirmed by Emirgil and correlated to symptoms of chronic bronchitis and shortness of breath in a similar group of alcoholics (Emirgil et al., 1974). A later study by Emirgil in 1977 studied pulmonary function in 44 abstinent members of Alcoholics Anonymous and found that 64% had airflow obstruction and 16% and 17% exhibited significant air trapping and/or impaired diffusion, respectively (Emirgil and Sobol, 1977). Airflow obstruction could not be accounted for on the basis of current smoking status or previous infection. Noteworthy in this small study was the high incidence of airflow obstruction in women (77%). The authors asserted that these findings suggest that alcohol per se impairs lung function. This conclusion was hampered by the small study size, the focus only on alcoholics, and their comparison to historical controls. In a case-control study, Lyons performed pulmonary function tests and assessed respiratory symptoms on 27 alcoholic subjects and case-matched control subjects (Lyons et al., 1986). They found there was no difference in pulmonary function or symptoms between the two groups and could account for all abnormal function on the basis of smoking alone. A subsequent study of 111 alcoholics and controls by Garshick found that lifetime alcohol consumption was a predictor of chronic cough and sputum production but not wheeze (Garshick et al., 1989). Using a linear regression model that included age, smoking history measured in pack/years, and interactions between pack/years and alcohol intake, Garshick found that lifetime alcohol consumption was also a predictor of lower FEV1 on spirometry. Interestingly, they found that the interaction between alcohol and smoking consumption was in a direction opposite to the independent effects of alcohol and smoking on lung function and suggested that alcohol might exert a protective effect in heavy smokers. This study demonstrates the challenge of dealing with smoking and other environmental factors that must be considered when trying to link alcohol intake to a disease with multifactorial exposures. Studies of twins often shed light on the interplay between genetic and environmental exposures.
Hrubec and colleagues used the US Twin Registry to determine which environmental factors were associated with respiratory symptoms in 4,388 adult male twin pairs who were also veterans (Hrubec et al., 1973). Prolonged cough and bronchitis were highly associated with smoking, drinking alcohol, and poor socioeconomic status. Alcohol intoxication, independent of smoking status, was associated with a two-fold increased risk for prolonged cough or bronchitis. Importantly, no significant difference was observed between members of twin pairs suggesting that environmental exposure rather than genetics played a major role in developing these respiratory symptoms associated with COPD. A limitation of this study was the exclusion of women and a lack of pulmonary function assessment.
The first large population study that examined the relationship of alcohol consumption to airway obstruction was a cross-sectional analysis published by Cohen in 1980 (Cohen et al., 1980). This study used data from a cohort of 2,539 community dwelling adults that quantified alcohol intake, smoking, diet and other health factors and measured FEV1 on spirometry. Although unadjusted values indicated obstruction in heavy drinkers compared to light drinkers, the difference disappeared when adjustment was made for cigarette smoking, socioeconomic status, male sex and age. They concluded that there is no evidence for an independent association of alcohol intake on airflow obstruction.
A second population study by Lebowitz surveyed symptoms of cough, wheeze and dyspnea, measured pulmonary function and captured physician-confirmed diagnoses of respiratory disease in 3,800 subjects in the Tucson Arizona area (Lebowitz, 1981). He found that alcohol consumption was associated with increased symptoms and decreased pulmonary function independent of smoking status. Interestingly, this study found the same relationship of alcohol intake with symptoms and function changes in women, although the effects of alcohol were more prominent in men. Importantly, they noted that this adverse pulmonary association with alcohol intake remained strong when they restricted the analysis to men that had never smoked. While the author recognized that smoking was the major factor associated with symptoms and pulmonary function change, alcohol represented a distinct and significant risk factor.
A third population study was both a cross-sectional and longitudinal analysis of 1,067 male veterans during a 5-year period (Sparrow et al., 1983). They found that alcohol consumption was not associated with changes in pulmonary function measured by spirometry when confounding values were taken into consideration. Similarly, alcohol intake did not influence changes in function on 5-year follow-up. The major limitation of this study was that it excluded women. The authors also recognized that pulmonary function measurements do not correlate well with patient function and symptoms.
The negative pulmonary function studies did not, however, put the issue to rest. Several years later Lange, in a larger and longitudinal population study from Copenhagen, examined 8,765 persons over five years with alcohol intake histories, smoking histories and pulmonary function tests (Lange et al., 1988). Using multiple regression analysis, these investigators found that alcohol consumption significantly accelerated the loss of FEV1 and vital capacity over time. To graphically illustrate their point they equated the impact of drinking 350 grams of alcohol per week (about thirty 12-ounce beers or thirty 5-ounce glasses of wine or 45 ounces of distilled spirits) to smoking 15 grams of tobacco (1 gram = 1 cigarette or 1/5th of a cigar) per day. The distinct advantages of this study were the longitudinal and prospective nature of the data collection and the quantitative intake data for alcohol and smoking that allowed for valid multiple regression analysis. To summarize, early population studies provide an important perspective on the role alcohol might play in the pathogenesis of COPD and indicate that alcohol intake either has no effect or may independently increase risk for developing COPD.
The most recent published population study examining the association of alcohol intake with pulmonary function utilized data from the Third National Health and Nutrition Examination Survey (NHANES III; (1994), to compare the prevalence of airway obstruction on spirometry with alcohol intake (Sisson et al., 2005). The NHANES III dataset has alcohol intake and matching pulmonary function data from 15,294 adults that represent the population of the United States between 1988 and 1994. This analysis failed to demonstrate reduced risk in subjects with mild alcohol consumption, but did demonstrate increased odds for airflow obstruction in former heavy drinkers. This association was independent of age, sex, education, socioeconomic status and, importantly, smoke exposure. Unexpectedly, this study also found that alcohol consumption was associated with an alcohol intake-dependent reduction of odds for the presence of lung restriction, which confirmed an incidental observation made by Cohen in 1980 (Cohen et al., 1980). Lung restriction can occur from external compression of the lung, such as with obesity or chest wall deformity, from congestive heart failure and pulmonary edema or from intrinsic lung diseases such as idiopathic fibrosis or sarcoidosis. This is the largest population study to date, is significant for its representation of the entire US population, and it identifies former heavy drinkers as a subset of individuals at increased risk for developing COPD.
Another approach to the problem was taken by Umbricht-Schneiter who prospectively examined hospital records of 1,964 patients admitted for a variety of medical problems (Umbricht-Schneiter et al., 1991). The authors associated each hospitalization to alcohol abuse (or not) by either capturing an alcohol-related diagnosis in the medical record or by two standardized alcohol abuse questionnaires administered to each patient. Combining both methods, they found an overall prevalence of an alcohol-related diagnosis (ARD) of 22.4% in all hospitalized patients and found that the diagnoses recorded in the chart identified only one-third of the patients with a current history of alcohol abuse. Regardless of how patients were linked to an ARD, they had an increased risk for COPD during that hospitalization. This study is important because it determined how frequently alcohol abuse goes unnoticed in hospitalized patients and how often that COPD is associated with such admissions. While this approach complements many of the other studies linking heavy alcohol intake to COPD, there are also studies that assert that alcohol intake may protect from the development of COPD.
A controversial autopsy survey was the first to assert that alcohol consumption might confer a protective effect against the development of COPD. Pratt and colleagues compared the morphometric analysis of 204 autopsied lungs from normal patients, patients with clinically recognized emphysema, and patients with other illnesses to alcohol intake histories available on these patients (Pratt and Vollmer, 1984). The authors found that alcohol use was significantly associated with reduced centrilobular emphysema even after adjusting for age and smoking effects. They speculated that the difference was due to inhibition of inflammatory cells in the lungs. These provocative findings prompted a heated debate about the broad applicability of small statistical studies to clinical practice, the difference between association and causality, and the challenge inherent to quantifying cigarette smoking in heavy drinkers (Hansen, 1984). When considered in the context of the population studies, this study also demonstrates the difficulty in equating symptoms, pulmonary function measurements and pathologic findings in lung tissues (Pratt and Vollmer, 1988). Regardless of these potentially important distinctions, this interesting autopsy study first raised the possibility that alcohol could potentially have a protective rather than contributive impact of the pathophysiology of COPD.
Two epidemiologic studies from Europe lend credence to the hypothesis that alcohol intake may reduce the risk for COPD. Because alcohol consumption shows a U-shaped curve with cardiovascular mortality (Murray et al., 2002; Rimm et al., 1991), these investigators hypothesized a similar relation between alcohol consumption and COPD mortality. The first study compared twenty-year COPD mortality and pulmonary function to alcohol consumption in three European countries (Tabak et al., 2001b). Analysis of data from 2,953 middle aged men from Finland, Italy and the Netherlands showed reduced COPD mortality in mild drinkers compared to non-drinkers (relative risk of 0.60). In contrast to mild drinkers, COPD mortality was increased in heavy-to-moderate drinkers (relative risk of 1.25). A similar U-shaped risk curve for reduced pulmonary function was observed among non-drinkers, mild drinkers and moderate-to-heavy drinkers. Importantly, the U-shaped risk curve was independent of age, height, body mass index (BMI), smoking status, energy intake or country. A second analysis by these same investigators of patients in the Netherlands demonstrated independent beneficial effects of moderate alcohol intake and consumption of foods high in antioxidants such as fruits and whole grains on the risk for COPD symptoms and pulmonary function (Tabak et al., 2001a). Taken together, these studies are the first to link mild alcohol intake to reduced risk for developing or dying from COPD, and are consistent with the controversial autopsy findings of Pratt three decades earlier (Pratt and Vollmer, 1984). The cause of mortality in these studies was not determined although an older study showed that acute ingestion of alcohol increased the incidence of ventricular ectopy and apnea in COPD patients (Dolly and Block, 1983).
Summary of Alcohol and COPD
It is challenging to draw firm conclusions from the divergent results of COPD studies and alcohol over the past five decades. A few themes do emerge: 1) heavy alcohol intake likely exacerbates smoking-related risk for COPD; 2) mild alcohol intake may reduce the risks of dying from COPD and developing severe pulmonary function abnormalities in COPD patients; 3) great care must be taken to separate smoking exposure in any analysis of alcohol and COPD; 4) the Breathalyzer test is not valid in patients with severe COPD for estimating blood alcohol levels; 5) as is true with COPD in general, the association of disease symptoms with impaired pulmonary function is poor and need to be independently assessed in any study that studies the impact of alcohol intake in COPD patients; and 6) unlike studies of alcohol and asthma, there are virtually no basic science studies of alcohol and COPD. This is due to the long time required for emphysema and/or airway disease to develop in animal models of COPD and limits our understanding of the role and potential mechanisms that link alcohol to COPD.
Overall Summary
The volatility of alcohol promotes the movement of alcohol from the bronchial circulation across the airway epithelium and into the conducting airways of the lung. The exposure of the airways through this route likely accounts for many of the biologic effects of alcohol on lung airway functions. The impact of alcohol on lung airway functions is dependent on the concentration, duration and route of exposure.
Brief exposure to mild concentrations of alcohol may enhance mucociliary clearance, stimulates bronchodilation and probably attenuates the airway inflammation and injury observed in asthma and COPD. Prolonged and heavy exposure to alcohol impairs mucociliary clearance, may complicate asthma management and likely worsens outcomes including lung function and mortality in COPD patients. Non-alcohol congeners and alcohol metabolites act as triggers for airway disease exacerbations especially in atopic asthmatics and in Asian populations who have a reduced capacity to metabolize alcohol.
Research focused on the mechanisms of alcohol-mediated changes in airway functions has identified specific mechanisms that mediate alcohol effects within the lung airways. These include prominent roles for the second messengers calcium and nitric oxide, regulatory kinases including PKG and PKA, alcohol and aldehyde -metabolizing enzymes such as ALDH2. The role alcohol may play in the biology of airway mucus, bronchial blood flow, airway smooth muscle regulation and the interaction with other airway exposure agents, such as cigarette smoke, represent opportunities for future investigation.
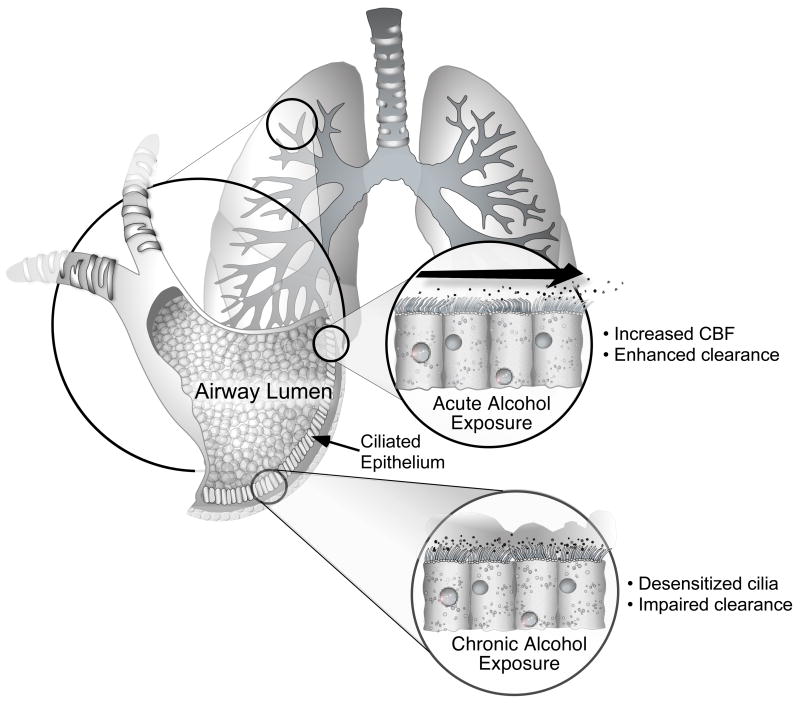
Figure 1. Acute and chronic effects of alcohol on airway cilia function.
Upper panel: Brief exposure to modest concentrations of alcohol stimulates airway ciliated cell nitric oxide synthase (NOS) to produce nitric oxide (NO), activate guanylyl cyclase (GC) to produce cGMP, which activates a cGMP-dependent kinase (PKG) to phosphorylate a specific ciliary protein (pp28). In parallel, alcohol activates adenylyl cyclase isoform 7 (AC7) to produce cAMP, which activates a cAMP-dependent kinase (PKA) to phosphorylate another specific ciliary protein (pp29). The dual activation of these kinases by alcohol results in stimulation of ciliary beat frequency (CBF).
Lower panel: Prolonged exposure to high concentrations of alcohol stimulates airway ciliated cell phosphodiesterase isoform 4 (PDE4) to degrade cAMP reducing ciliary beat frequency to pre-alcohol exposure levels. Concomitantly, alcohol desensitizes both the cGMP-dependent kinase (PKG) and the cAMP-dependent kinase (PKA) rendering these kinases resistant to activation. This dual kinase desensitization results in a the ciliated cell that is unresponsive to stimulation with beta agonists.
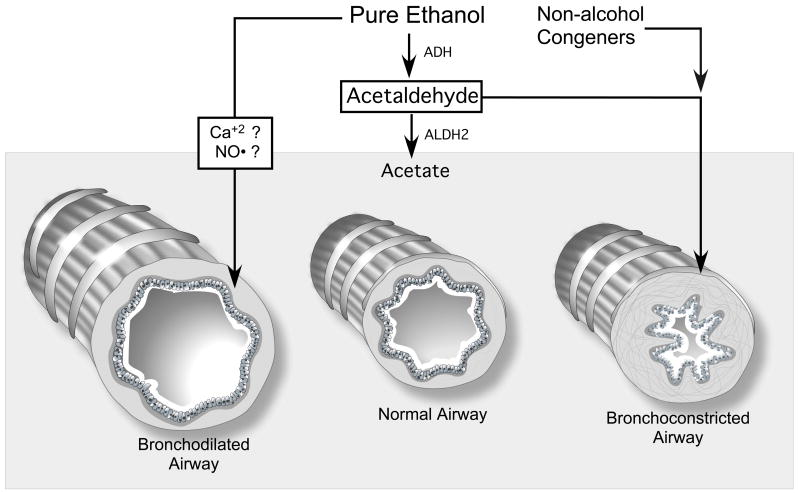
Figure 2. Effects of alcohol and related molecules on bronchial motor tone.
Alcohol (pure ethanol), in the absence of any metabolites or congeners, relaxes airway smooth muscle tone resulting in bronchodilated airways. Non-alcohol congeners, often present in alcoholic beverages, can cause contraction of airway smooth muscle resulting in bronchoconstricted airways in some sensitized or allergic individuals. Acetaldehyde, the product of alcohol metabolism, can accumulate in individuals with genetically reduced aldehyde dehydrogenase isoform 2 deficiency (ALHD2), causing in bronchoconstricted airways resulting in “alcohol-induced bronchial asthma” (Shimoda et al., 1996).
Table 1.
The effects of alcohol on mucociliary function (also see Figure 1)
| Acute Effects | Chronic Effects | ||
|---|---|---|---|
| Observation | Reference | Observation | Reference |
Ciliostasis
|
(Dalhamn et al., 1967; Nungester and Klepser, 1938; Purkinje and Valentine, 1835) |
Clearance faster
|
(Venizelos et al., 1981) |
Ciliary beating stimulated
|
(Maurer and Liebman, 1988; Sisson, 1995; Sisson et al., 1999; Wyatt et al., 2003; Wyatt et al., 2004) |
Cilia kinases desensitized
|
(Forget et al., 2003; Wyatt et al., 2004)
(Forget et al., 2003) |
Clearance slowed
|
(Laurenzi and Guarneri, 1966) |
Cilia motility desensitized
|
(Wyatt and Sisson, 2001) |
Table 2.
Effects of alcohol on asthma and airflow
| Bronchoconstriction | Bronchodilation | ||
|---|---|---|---|
| Clinical Findings | |||
| Effect | Reference | Effect | Reference |
| Atopic patients wheeze from alcohol congeners but not pure alcohol | (Breslin et al., 1973) | Alcohol ingestion improves vital capacity | (Herxheimer and Stresemann, 1963) |
| Nebulized alcohol triggers coughing and reduction in airflow in healthy volunteers | (Zuskin et al., 1981) | Alcohol triggers an immediate irritant response and causes a slow, bronchodilator effect | (Ayres et al., 1982) |
| Sulfur dioxide in some wines cause wheezing in asthmatics | (Dahl et al., 1986) | Alcohol administered IV acts as a bronchodilator | (Brown, 1947) |
| Acetaldehyde causes “alcohol- induced bronchial asthma” in some Asians | (Shimoda et al., 1996) | Alcohol administered IV acts as a concentration-dependent bronchodilator | (Ayres and Clark, 1983b) |
| Aldehyde dehydrogenase type-2 (ALHD2) deficiency identified as cause of asthma some Asians | (Matsuse et al., 2001) | ||
| Basic Science Findings | |||
| Effect | Reference | Effect | Reference |
| Alcohol causes concentration- dependent contraction of airway smooth muscle in guinea pigs | (Jakupi et al., 1986) | Alcohol blocks ovalbumin-triggered bronchoconstriction | (Fleisch et al., 1976) |
| Alcohol triggers airway inflammation and smooth muscle contraction | (Trevisani et al., 2004) | High concentrations of ethanol inhibit antigen-induced histamine release | (Mongar and Schild, 1957) |
| Very high concentrations of ethanol block calcium uptake and binding of calcium to cardiac microsomes | (Swartz et al., 1974) | ||
| Alcohol and acetaldehyde, inhibit adenosine monophosphate-induced re- association of skeletal actin and myosin | (Puszkin and Rubin, 1975) | ||
| Alcohol causes hyper polarization and suppression of membrane action potentials in canine tracheal smooth muscle | (Richards et al., 1989) | ||
| Alcohol promotes concentration- dependent airway smooth muscle relaxation and increased sensitivity to calcium | (Hanazaki et al., 2001) | ||
Acknowledgments
Dr. Sisson gratefully acknowledges Lisa Chudomelka for her help with the research and the preparation of this manuscript and Arthur Heires for his help creating the figures. Dr. Sisson is supported by the National Institute on Alcohol Abuse and Alcoholism (Grant NIAAA 2R01AA008769-15A).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Plan operation of the Third National Health Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures Vital Health Stat 1. 1994:1–407. [PubMed] [Google Scholar]
- AMA. Referendum on the use of alcohol in the practice of medicine. Final Report JAMA. 1922;75:210. [Google Scholar]
- Antonicelli L, Micucci C, Bonifazi F. Bronchospasm induced by inhalant corticosteroids: the role of ethanol. Allergy. 2006a;61:146–147. doi: 10.1111/j.1398-9995.2005.00943.x. [DOI] [PubMed] [Google Scholar]
- Antonicelli L, Micucci C, Bonifazi F. Is ethanol-induced bronchospasm an inflammation-driven event? Allergy. 2006b;61:270–271. doi: 10.1111/j.1398-9995.2006.01003.x. [DOI] [PubMed] [Google Scholar]
- Ayres J, Ancic P, Clark TJ. Airways responses to oral ethanol in normal subjects and in patients with asthma. J R Soc Med. 1982;75:699–704. doi: 10.1177/014107688207500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres JG. The history of the use of alcohol in the treatment of respiratory diseases. Br J Dis Chest. 1987;81:80–86. doi: 10.1016/0007-0971(87)90112-4. [DOI] [PubMed] [Google Scholar]
- Ayres JG, Clark TJ. Alcoholic drinks and asthma: a survey. Br J Dis Chest. 1983a;77:370–375. [PubMed] [Google Scholar]
- Ayres JG, Clark TJ. Intravenous ethanol can provide bronchodilatation in asthma. Clin Sci (Lond) 1983b;64:555–557. doi: 10.1042/cs0640555. [DOI] [PubMed] [Google Scholar]
- Badham C. An essay on bronchiolitis: with a supplement containing remarks on simple pulmonary abscess (England) 1814 [Google Scholar]
- Banner AS. Pulmonary function in chronic alcoholism. Am Rev Respir Dis. 1973;108:851–857. doi: 10.1164/arrd.1973.108.4.851. [DOI] [PubMed] [Google Scholar]
- Bomalaski JS, Phair JP. Alcohol, immunosuppression, and the lung. Arch Intern Med. 1982;142:2073–2074. [PubMed] [Google Scholar]
- Boyd EM, Sheppard EP. Expectorant action of inhaled alcohol. Arch Otolaryngol. 1969;90:374–379. doi: 10.1001/archotol.1969.00770030376021. [DOI] [PubMed] [Google Scholar]
- Breslin AB, Hendrick DJ, Pepys J. Effect of disodium cromoglycate on asthmatic reactions to alcoholic beverages. Clin Allergy. 1973;3:71–82. doi: 10.1111/j.1365-2222.1973.tb01311.x. [DOI] [PubMed] [Google Scholar]
- Brown EA. The use of intravenous ethyl alcohol in the treatment of status asthmaticus. Ann Allergy. 1947;5:193–195. [PubMed] [Google Scholar]
- Burch GE, DePasquale NP. Alcoholic lung disease--an hypothesis. Am Heart J. 1967;73:147–148. doi: 10.1016/0002-8703(67)90140-8. [DOI] [PubMed] [Google Scholar]
- Chomet B, Gach BM. Lobar pneumonia and alcoholism: an analysis of thirty-seven cases. Am J Med Sci. 1967;253:300–304. doi: 10.1097/00000441-196703000-00006. [DOI] [PubMed] [Google Scholar]
- Chu I, Poon R, Valli V, Yagminas A, Bowers WJ, Seegal R, Vincent R. Effects of an ethanol-gasoline mixture: results of a 4-week inhalation study in rats. J Appl Toxicol. 2005;25:193–199. doi: 10.1002/jat.1051. [DOI] [PubMed] [Google Scholar]
- Cohen BH, Celentano DD, Chase GA, Diamond EL, Graves CG, Levy DA, Menkes HA, Meyer MB, Permutt S, Tockman MS. Alcohol consumption and airway obstruction. Am Rev Respir Dis. 1980;121:205–215. doi: 10.1164/arrd.1980.121.2.205. [DOI] [PubMed] [Google Scholar]
- Dahl R, Henriksen JM, Harving H. Red wine asthma: a controlled challenge study. J Allergy Clin Immunol. 1986;78:1126–1129. doi: 10.1016/0091-6749(86)90261-7. [DOI] [PubMed] [Google Scholar]
- Dalhamn T, Holma B, Tomenius L. In vitro studies of the ciliotoxic action of ethanol vapor in relation to its concentration in tracheal tissue. Acta Pharmacol Toxicol (Copenh) 1967;25:272–280. doi: 10.1111/j.1600-0773.1967.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Dolly FR, Block AJ. Increased ventricular ectopy and sleep apnea following ethanol ingestion in COPD patients. Chest. 1983;83:469–472. doi: 10.1378/chest.83.3.469. [DOI] [PubMed] [Google Scholar]
- Dulfano MJ, Luk CK, Beckage M, Wooten O. Ciliary beat frequency in human respiratory explants. Am Rev Respir Dis. 1981;123:139–140. doi: 10.1164/arrd.1981.123.1.139. [DOI] [PubMed] [Google Scholar]
- Elliott MK, Sisson JH, Wyatt TA. Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment. Am J Respir Cell Mol Biol. 2007;36:452–459. doi: 10.1165/rcmb.2005-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emirgil C, Sobol BJ. Pulmonary function in former alcoholics. Chest. 1977;72:45–51. doi: 10.1378/chest.72.1.45. [DOI] [PubMed] [Google Scholar]
- Emirgil C, Sobol BJ, Heymann B, Shibutani K. Pulmonary function in alcoholics. Am J Med. 1974;57:69–77. doi: 10.1016/0002-9343(74)90770-0. [DOI] [PubMed] [Google Scholar]
- Fleisch JH, Calkins PJ, Troxell TC, Hooker CS. Inhibition of antigen-induced mediator release from guinea pig lung by alcohols. Am Rev Respir Dis. 1976;114:1107–1112. doi: 10.1164/arrd.1976.114.6.1107. [DOI] [PubMed] [Google Scholar]
- Forget MA, Sisson JH, Spurzem JR, Wyatt TA. Ethanol increases phosphodiesterase 4 activity in bovine bronchial epithelial cells. Alcohol. 2003;31:31–38. doi: 10.1016/j.alcohol.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Garshick E, Segal MR, Worobec TG, Salekin CM, Miller MJ. Alcohol consumption and chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989;140:373–378. doi: 10.1164/ajrccm/140.2.373. [DOI] [PubMed] [Google Scholar]
- George SC, Hlastala MP, Souders JE, Babb AL. Gas exchange in the airways. J Aerosol Med. 1996;9:25–33. doi: 10.1089/jam.1996.9.25. [DOI] [PubMed] [Google Scholar]
- Geppert EF, Boushey HA. An investigation of the mechanism of ethanol-induced bronchoconstriction. Am Rev Respir Dis. 1978;118:135–139. doi: 10.1164/arrd.1978.118.1.135. [DOI] [PubMed] [Google Scholar]
- Gong H, Jr, Tashkin DP, Calvarese BM. Alcohol-induced bronchospasm in an asthmatic patient: pharmacologic evaluation of the mechanism. Chest. 1981;80:167–173. doi: 10.1378/chest.80.2.167. [DOI] [PubMed] [Google Scholar]
- Hanazaki M, Jones KA, Perkins WJ, Warner DO. The effects of ethanol on CA(2+) sensitivity in airway smooth muscle. Anesth Analg. 2001;92:767–774. doi: 10.1097/00000539-200103000-00040. [DOI] [PubMed] [Google Scholar]
- Hansen JE. Alcohol consumption and emphysema. Chest. 1984;86:804. doi: 10.1378/chest.86.5.804-a. [DOI] [PubMed] [Google Scholar]
- Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2:428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- Heinemann HO. Alcohol and the lung. A brief review Am J Med. 1977;63:81–85. doi: 10.1016/0002-9343(77)90120-6. [DOI] [PubMed] [Google Scholar]
- Herxheimer H, Stresemann E. Ethanol and Lung Function in Bronchial Asthma. Arch Int Pharmacodyn Ther. 1963;144:310–314. [PubMed] [Google Scholar]
- Hlastala MP. The alcohol breath test--a review. J Appl Physiol. 1998;84:401–408. doi: 10.1152/jappl.1998.84.2.401. [DOI] [PubMed] [Google Scholar]
- Högman M, Frostell CG, Hedenström H, Hedenstierna G. Inhalation of nitric oxide modulates adult human bronchial tone. Am Rev Respir Dis. 1993;148:1474–1478. doi: 10.1164/ajrccm/148.6_Pt_1.1474. [DOI] [PubMed] [Google Scholar]
- Honeybourne D, Moore AJ, Butterfield AK, Azzan L. A study to investigate the ability of subjects with chronic lung diseases to provide evidential breath samples using the Lion Intoxilyzer 6000 UK breath alcohol-testing device. Respir Med. 2000;94:684–688. doi: 10.1053/rmed.2000.0797. [DOI] [PubMed] [Google Scholar]
- Hooper G, Steed KP, Gittins DP, Newman SP, Richards A, Rubin I. Bronchoconstriction following inhaled ethanol solutions. Respir Med. 1995;89:457–458. doi: 10.1016/0954-6111(95)90221-x. [DOI] [PubMed] [Google Scholar]
- Hrubec Z, Cederlof R, Friberg L, Horton R, Ozolins G. Respiratory symptoms in twins: effects of residence-associated air pollution, tobacco and alcohol use, and other factors. Arch Environ Health. 1973;27:189–195. doi: 10.1080/00039896.1973.10666350. [DOI] [PubMed] [Google Scholar]
- Hutchinson J. On the capacity of the lungs, and on the respiratory movements, with a view of establishing a precise and easy method of detecting disease by the spirometer. Med Chir Tr. 1846;29:137–161. doi: 10.1177/095952874602900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakupi M, Djokic TD, Karahoda-Gjurgjeala N, Zuskin E, Haxhiu MA. Effect of ethanol on the isolated airway smooth muscle tone. Acta Med Iugosl. 1986;40:207–214. [PubMed] [Google Scholar]
- Jalleh R, Fitzpatrick MF, Jan MA, MacNee W, Douglas NJ. Alcohol and cor pulmonale in chronic bronchitis and emphysema. B M J. 1993;306:374. doi: 10.1136/bmj.306.6874.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korri UM, Salaspuro M. Increased rate of ethanol elimination and elevated blood acetate in asthmatics on corticosteroid, beta-2–sympathicomimetic and theophylline treatment. Alcohol Alcohol. 1988;23:371–376. doi: 10.1093/oxfordjournals.alcalc.a044831. [DOI] [PubMed] [Google Scholar]
- Lange P, Groth S, Mortensen J, Appleyard M, Nyboe J, Jensen G, Schnohr P. Pulmonary function is influenced by heavy alcohol consumption. Am Rev Respir Dis. 1988;137:1119–1123. doi: 10.1164/ajrccm/137.5.1119. [DOI] [PubMed] [Google Scholar]
- Laurenzi GA, Guarneri JJ. Effects of bacteria and viruses on ciliated epithelium. A study of the mechanisms of pulmonary resistance to infection: the relationship of bacterial clearance to ciliary and alveolar macrophage function. Am Rev Respir Dis. 1966;93(Suppl):134–141. doi: 10.1164/arrd.1966.93.3P2.134. [DOI] [PubMed] [Google Scholar]
- Laurenzi GA, Guarneri JJ, Endriga RB. Important Determinants in Pulmonary Resistance to Bacterial Infection. Med Thorac. 1965;22:48–59. [PubMed] [Google Scholar]
- Laurenzi GA, Guarneri JJ, Endriga RB, Carey JP. Clearance of Bacteria by the Lower Respiratory Tract. Science. 1963;142:1572–1573. doi: 10.1126/science.142.3599.1572. [DOI] [PubMed] [Google Scholar]
- Laurenzi GA, Potter RT, Kass EH. Bacteriologic flora of the lower respiratory tract. N Engl J Med. 1961;265:1273–1278. doi: 10.1056/NEJM196112282652601. [DOI] [PubMed] [Google Scholar]
- Leake C. The old Egyptian Medical Papyri. Lawrence, Kansas: University of Kansas Press; 1952. [Google Scholar]
- Lebowitz MD. Respiratory symptoms and disease related to alcohol consumption. Am Rev Respir Dis. 1981;123:16–19. doi: 10.1164/arrd.1981.123.1.16. [DOI] [PubMed] [Google Scholar]
- Leffman H. N Y Med J. 1885;42:21. [Google Scholar]
- Leitch GJ, Frid LH, Phoenix D. The effects of ethanol on mucociliary clearance. Alcohol Clin Exp Res. 1985;9:277–280. doi: 10.1111/j.1530-0277.1985.tb05749.x. [DOI] [PubMed] [Google Scholar]
- Lucia S. A history of wine as therapy. Montreal, Canada: Lippincott Co; 1963. [Google Scholar]
- Lyons DJ, Howard SV, Milledge JS, Peters TJ. Contribution of ethanol and cigarette smoking to pulmonary dysfunction in chronic alcoholics. Thorax. 1986;41:197–202. doi: 10.1136/thx.41.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58:388–393. doi: 10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuse H, Shimoda T, Fukushima C, Mitsuta K, Kawano T, Tomari S, Saeki S, Kondoh Y, Machida I, Obase Y, et al. Screening for acetaldehyde dehydrogenase 2 genotype in alcohol-induced asthma by using the ethanol patch test. J Allergy Clin Immunol. 2001;108:715–719. doi: 10.1067/mai.2001.118791. [DOI] [PubMed] [Google Scholar]
- Maurer DR, Liebman J. Effects of ethanol on in vitro ciliary motility. J Appl Physiol. 1988;65:1617–1620. doi: 10.1152/jappl.1988.65.4.1617. [DOI] [PubMed] [Google Scholar]
- Mongar JL, Schild HO. Inhibition of the anaphylactic reaction. J Physiol. 1957;135:301–319. doi: 10.1113/jphysiol.1957.sp005712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RP, Connett JE, Tyas SL, Bond R, Ekuma O, Silversides CK, Barnes GE. Alcohol volume, drinking pattern, and cardiovascular disease morbidity and mortality: is there a U-shaped function? Am J Epidemiol. 2002;155:242–248. doi: 10.1093/aje/155.3.242. [DOI] [PubMed] [Google Scholar]
- Myou S, Fujimura M, Bando T, Saito M, Matsuda T. Aerosolized acetaldehyde, but not ethanol, induces histamine-mediated bronchoconstriction in guinea pigs. Clin Exp Allergy. 1994;24:140–143. doi: 10.1111/j.1365-2222.1994.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Myou S, Fujimura M, Nishi K, Watanabe K, Matsuda M, Ohka T, Matsuda T. Effect of ethanol on airway caliber and nonspecific bronchial responsiveness in patients with alcohol-induced asthma. Allergy. 1996;51:52–55. doi: 10.1111/j.1398-9995.1996.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M, Zariwala MA, Knowles MR. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169:459–467. doi: 10.1164/rccm.200303-365OC. [DOI] [PubMed] [Google Scholar]
- Nungester WJ, Klepser RG. A possible mechanism of lowered resistance to pneumonia. J Infect Dis. 1938;63:94–102. [Google Scholar]
- Persson MG, Gustafsson LE. Ethanol can inhibit nitric oxide production. Eur J Pharmacol. 1992;224:99–100. doi: 10.1016/0014-2999(92)94826-h. [DOI] [PubMed] [Google Scholar]
- Petty TL. COPD in perspective. Chest. 2002;121:116S–120S. doi: 10.1378/chest.121.5_suppl.116s. [DOI] [PubMed] [Google Scholar]
- Pratt PC, Vollmer RT. The beneficial effect of alcohol consumption on the prevalence and extent of centrilobular emphysema. A retrospective autopsy analysis. Chest. 1984;85:372–377. doi: 10.1378/chest.85.3.372. [DOI] [PubMed] [Google Scholar]
- Pratt PC, Vollmer RT. Effect of alcohol consumption on emphysema or pulmonary function. Am Rev Respir Dis. 1988;138:1358–1359. doi: 10.1164/ajrccm/138.5.1358b. [DOI] [PubMed] [Google Scholar]
- Press A. Alcohol inhaling machine goes on display. USA Today; New York: 2004. [Google Scholar]
- Purkinje JE, Valentine GG. De phaenomeno generali et fundamentali motus vibratorii continui in membranis cum externus tum internis animalium plurimorum et superiorum et inferiorum ordinum obvio (Wratislaviae) 1835 [Google Scholar]
- Puszkin S, Rubin E. Adenosine diphosphate effect on contractility of human muscle actomyosin: inhibition by ethanol and acetaldehyde. Science. 1975;188:1319–1320. doi: 10.1126/science.124949. [DOI] [PubMed] [Google Scholar]
- Richards IS, Kulkarni AP, Brooks SM. Ethanol-induced bronchodilatation in TEA-treated canine tracheal smooth muscle is mediated by a beta-adrenoceptor-dependent mechanism. Eur J Pharmacol. 1989;167:155–160. doi: 10.1016/0014-2999(89)90757-7. [DOI] [PubMed] [Google Scholar]
- Richardson BW. Med Press and Circulation. 1881;31:2. [Google Scholar]
- Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- Russell JC, Jones RL. Breath ethyl alcohol concentration and analysis in the presence of chronic obstructive pulmonary disease. Clin Biochem. 1983;16:182–187. doi: 10.1016/s0009-9120(83)90243-6. [DOI] [PubMed] [Google Scholar]
- Salter H. On the treatment of asthmatic paroxysm by fulkl doses of alcohol. Lancet. 1863;ii:558–559. [Google Scholar]
- Saric M, Lucic-Palaic S, Horton RJ. Chronic nonspecific lung disease and alcohol consumption. Environ Res. 1977;14:14–21. doi: 10.1016/0013-9351(77)90061-5. [DOI] [PubMed] [Google Scholar]
- Shimoda T, Kohno S, Takao A, Fujiwara C, Matsuse H, Sakai H, Watanabe T, Hara K, Asai S. Investigation of the mechanism of alcohol-induced bronchial asthma. J Allergy Clin Immunol. 1996;97:74–84. doi: 10.1016/s0091-6749(96)70285-3. [DOI] [PubMed] [Google Scholar]
- Sisson JH. Ethanol stimulates apparent nitric oxide-dependent ciliary beat frequency in bovine airway epithelial cells. Am J Physiol. 1995;268:L596–600. doi: 10.1152/ajplung.1995.268.4.L596. [DOI] [PubMed] [Google Scholar]
- Sisson JH, May K, Wyatt TA. Nitric oxide-dependent ethanol stimulation of ciliary motility is linked to cAMP-dependent protein kinase (PKA) activation in bovine bronchial epithelium. Alcohol Clin Exp Res. 1999;23:1528–1533. [PubMed] [Google Scholar]
- Sisson JH, Stoner JA, Romberger DJ, Spurzem JR, Wyatt TA, Owens-Ream J, Mannino DM. Alcohol intake is associated with altered pulmonary function. Alcohol. 2005;36:19–30. doi: 10.1016/j.alcohol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Sotaniemi E, Isoaho R, Huhti E, Huikko M, Koivisto O. Increased clearance of ethanol from the blood of asthmatic patients. Ann Allergy. 1972;30:254–257. [PubMed] [Google Scholar]
- Sovijarvi AR, Hillbom ME, Poppius H. Effect of ethanol ingestion on blood gases and airway conductance in chronic bronchitis. Bull Eur Physiopathol Respir. 1978;14:409–415. [PubMed] [Google Scholar]
- Sparrow D, Rosner B, Cohen M, Weiss ST. Alcohol consumption and pulmonary function. A cross-sectional and longitudinal study. Am Rev Respir Dis. 1983;127:735–738. doi: 10.1164/arrd.1983.127.6.735. [DOI] [PubMed] [Google Scholar]
- Swartz MH, Repke DI, Katz AM, Rubin E. Effects of ethanol on calcium binding and calcium uptake by cardiac microsomes. Biochem Pharmacol. 1974;23:2369–2376. doi: 10.1016/0006-2952(74)90226-3. [DOI] [PubMed] [Google Scholar]
- Tabak C, Smit HA, Heederik D, Ocke MC, Kromhout D. Diet and chronic obstructive pulmonary disease: independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study) Clin Exp Allergy. 2001a;31:747–755. doi: 10.1046/j.1365-2222.2001.01064.x. [DOI] [PubMed] [Google Scholar]
- Tabak C, Smit HA, Rasanen L, Fidanza F, Menotti A, Nissinen A, Feskens EJ, Heederik D, Kromhout D. Alcohol consumption in relation to 20-year COPD mortality and pulmonary function in middle-aged men from three European countries. Epidemiology. 2001b;12:239–245. doi: 10.1097/00001648-200103000-00018. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Gazzieri D, Benvenuti F, Campi B, Dinh QT, Groneberg DA, Rigoni M, Emonds-Alt X, Creminon C, Fischer A, et al. Ethanol causes inflammation in the airways by a neurogenic and TRPV1-dependent mechanism. J Pharmacol Exp Ther. 2004;309:1167–1173. doi: 10.1124/jpet.103.064162. [DOI] [PubMed] [Google Scholar]
- Umbricht-Schneiter A, Santora P, Moore RD. Alcohol abuse: comparison of two methods for assessing its prevalence and associated morbidity in hospitalized patients. Am J Med. 1991;91:110–118. doi: 10.1016/0002-9343(91)90002-f. [DOI] [PubMed] [Google Scholar]
- Vally H, de Klerk N, Thompson PJ. Alcoholic drinks: important triggers for asthma. J Allergy Clin Immunol. 2000;105:462–467. doi: 10.1067/mai.2000.104548. [DOI] [PubMed] [Google Scholar]
- Vander Top EA, Wyatt TA, Gentry-Nielsen MJ. Smoke exposure exacerbates an ethanol-induced defect in mucociliary clearance of Streptococcus pneumoniae. Alcohol Clin Exp Res. 2005;29:882–887. doi: 10.1097/01.alc.0000164364.35682.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venizelos PC, Gerrity TR, Yeates DB. Response of human mucociliary clearance to acute alcohol administration. Arch Environ Health. 1981;36:194–201. doi: 10.1080/00039896.1981.10667625. [DOI] [PubMed] [Google Scholar]
- Verma M, Davidson EA. Transcriptional regulation of tracheo-bronchial mucin (TBM) gene by ethanol. Gene. 1997;189:9–12. doi: 10.1016/s0378-1119(96)00742-1. [DOI] [PubMed] [Google Scholar]
- Watanabe T. Mechanism of ethanol-induced bronchoconstriction in Japanese asthmatic patients. Arerugi. 1991;40:1210–1217. [PubMed] [Google Scholar]
- Wilson A, Sitar DS, Molloy WD, McCarthy D. Effect of age and chronic obstructive pulmonary disease on the Breathalyzer estimation of blood alcohol level. Alcohol Clin Exp Res. 1987;11:440–443. doi: 10.1111/j.1530-0277.1987.tb01919.x. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Forget MA, Sisson JH. Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am J Pathol. 2003;163:1157–1166. doi: 10.1016/S0002-9440(10)63475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TA, Gentry-Nielsen MJ, Pavlik JA, Sisson JH. Desensitization of PKA-stimulated ciliary beat frequency in an ethanol-fed rat model of cigarette smoke exposure. Alcohol Clin Exp Res. 2004;28:998–1004. doi: 10.1097/01.ALC.0000130805.75641.F4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TA, Sisson JH. Chronic ethanol downregulates PKA activation and ciliary beating in bovine bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L575–581. doi: 10.1152/ajplung.2001.281.3.L575. [DOI] [PubMed] [Google Scholar]
- Yates DH, Kharitonov SA, Robbins RA, Thomas PS, Barnes PJ. The effect of alcohol ingestion on exhaled nitric oxide. Eur Respir J. 1996;9:1130–1133. doi: 10.1183/09031936.96.09061130. [DOI] [PubMed] [Google Scholar]
- Zuskin E, Bouhuys A, Saric M. Lung function changes by ethanol inhalation. Clin Allergy. 1981;11:243–248. doi: 10.1111/j.1365-2222.1981.tb01590.x. [DOI] [PubMed] [Google Scholar]