Abstract
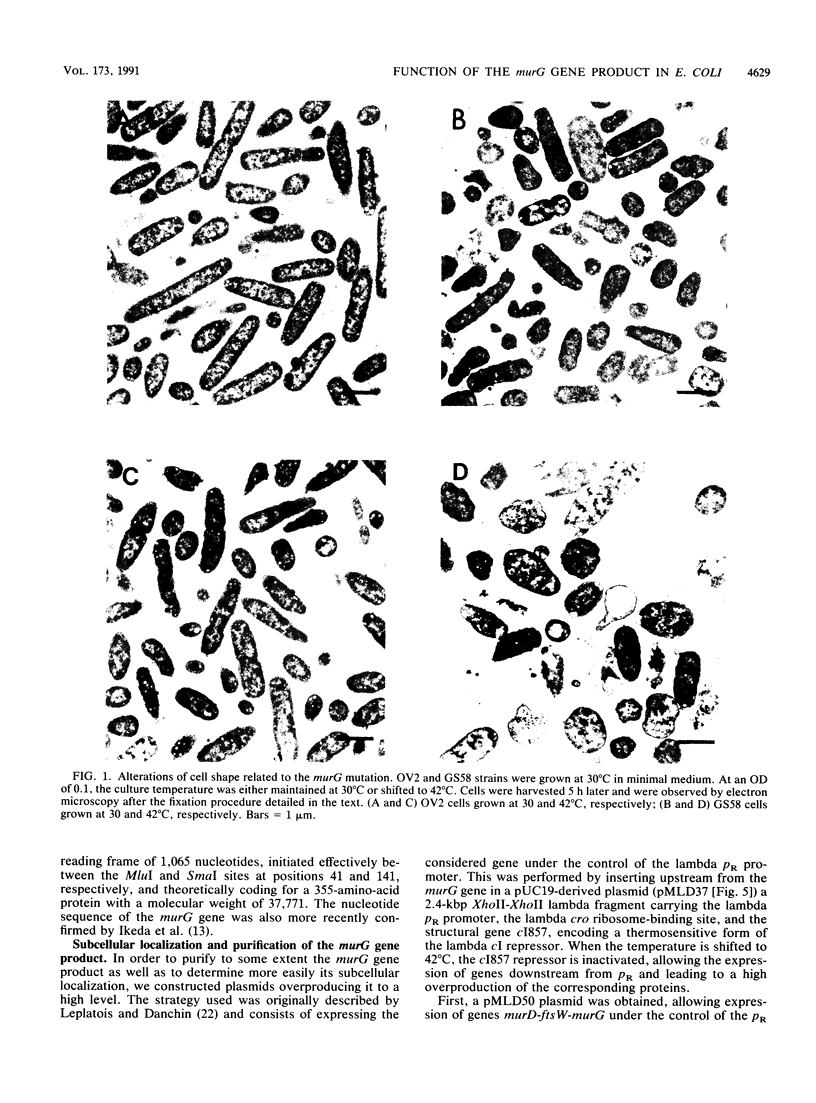
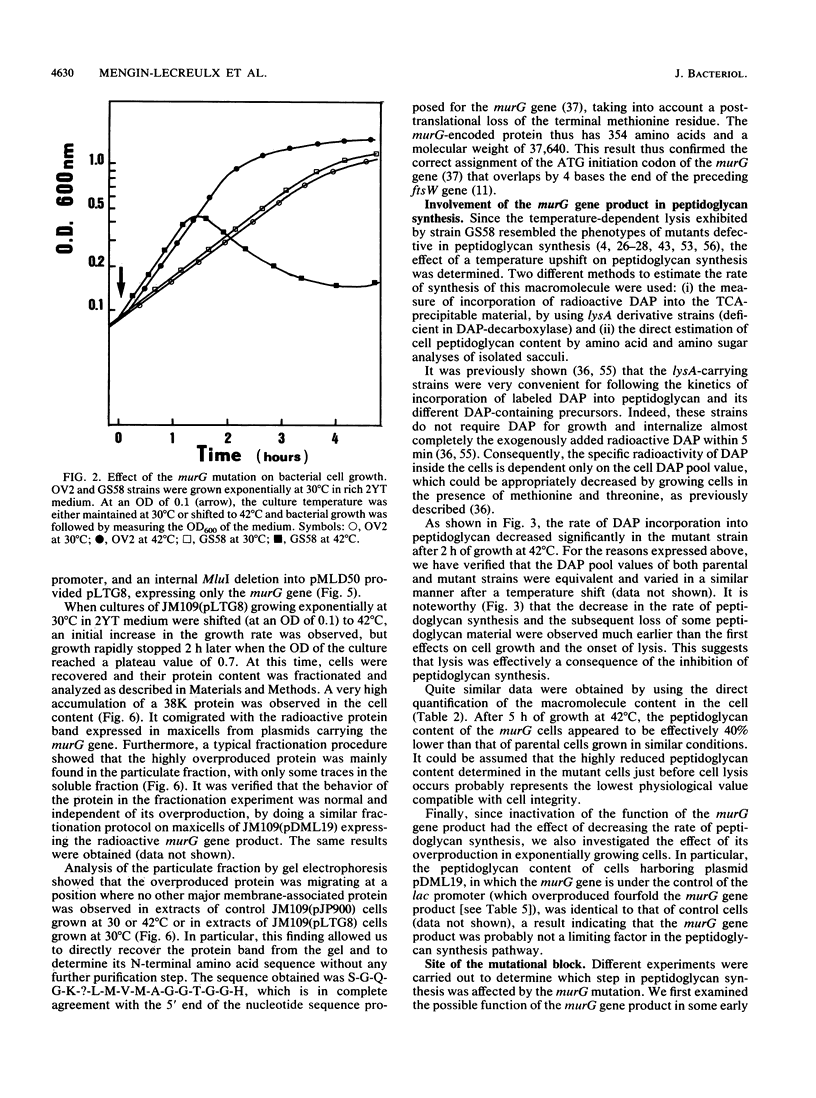
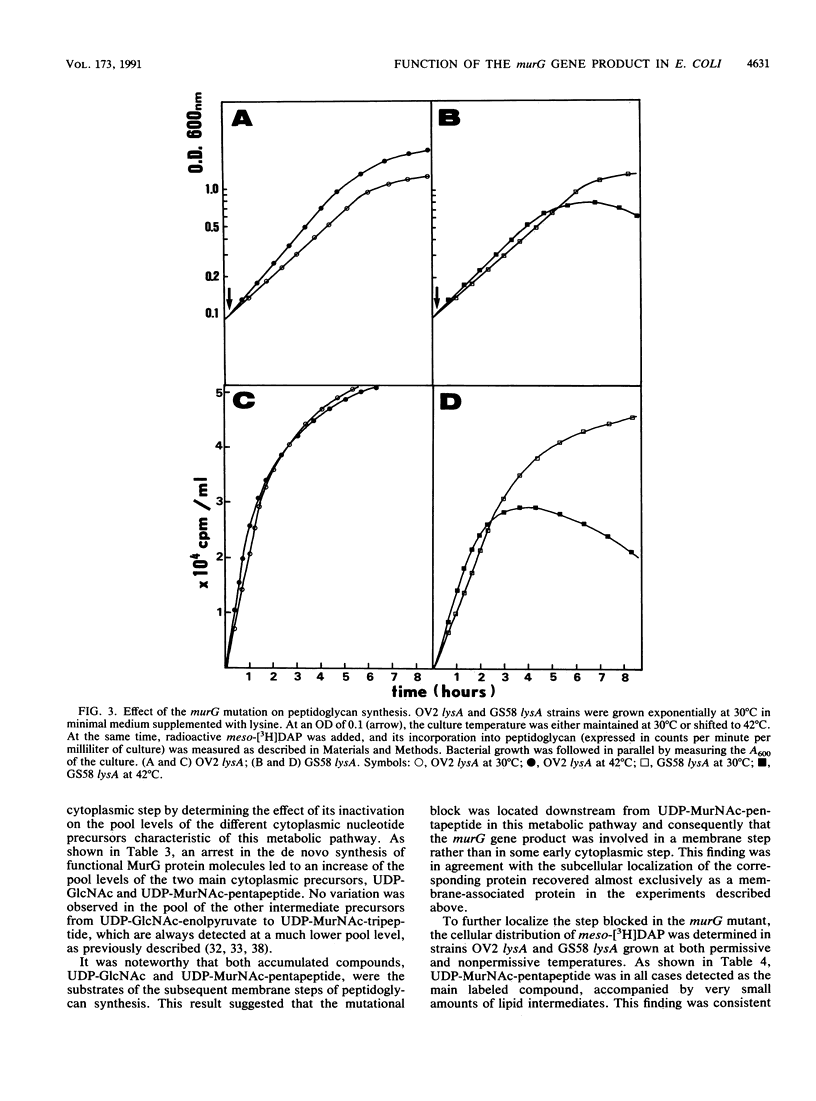
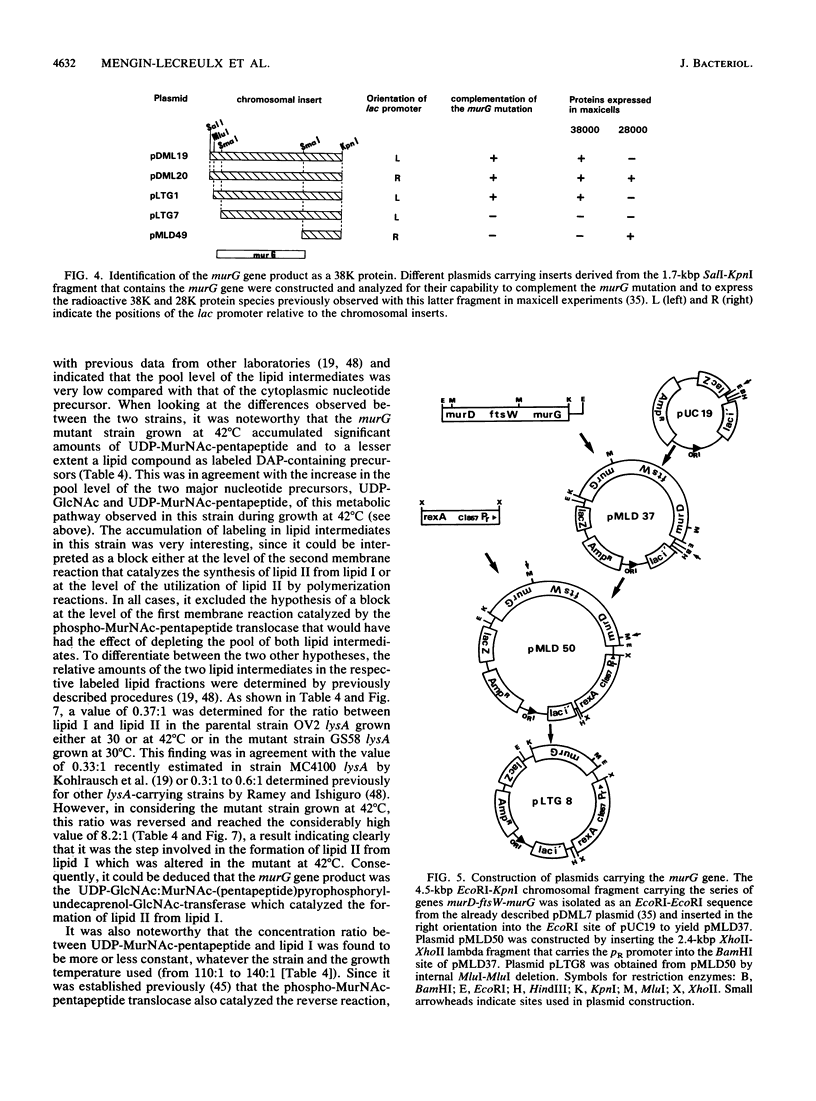
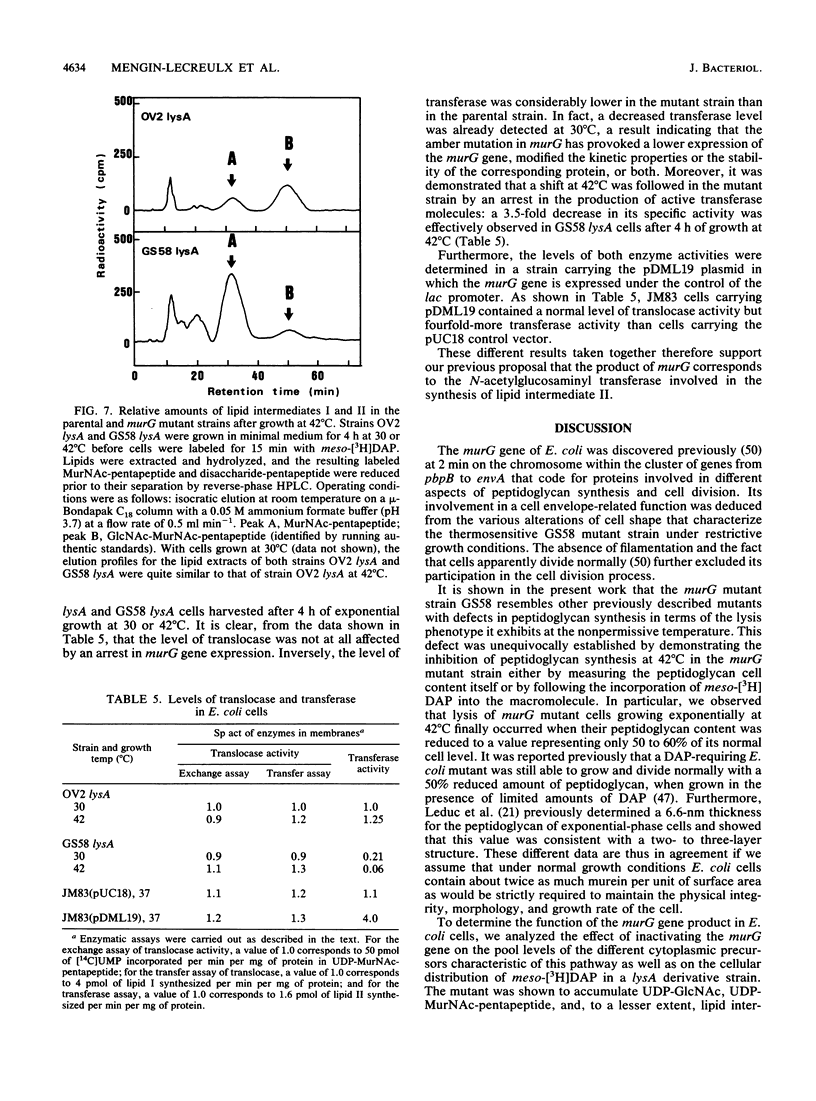
Physiological properties of the murG gene product of Escherichia coli were investigated. The inactivation of the murG gene rapidly inhibits peptidoglycan synthesis in exponentially growing cells. As a result, various alterations of cell shape are observed, and cell lysis finally occurs when the peptidoglycan content is 40% lower than that of normally growing cells. Analysis of the pools of peptidoglycan precursors reveals the concomitant accumulation of UDP-N-acetylglucosamine (UDP-GlcNAc) and UDP-N-acetylmuramyl-pentapeptide (UDP-MurNAc-pentapeptide) and, to a lesser extent, that of undecaprenyl-pyrophosphoryl-MurNAc-pentapeptide (lipid intermediate I), indicating that inhibition of peptidoglycan synthesis occurs after formation of the cytoplasmic precursors. The relative depletion of the second lipid intermediate, undecaprenyl-pyrophosphoryl-MurNAc-(pentapeptide)GlcNAc, shows that inactivation of the murG gene product does not prevent the formation of lipid intermediate I but inhibits the next reaction in which GlcNAc is transferred to lipid intermediate I. In vitro assays for phospho-MurNAc-pentapeptide translocase and N-acetylglucosaminyl transferase activities finally confirm the identification of the murG gene product as the transferase that catalyzes the conversion of lipid intermediate I to lipid intermediate II in the peptidoglycan synthesis pathway. Plasmids allowing for a high overproduction of the transferase and the determination of its N-terminal amino acid sequence were constructed. In cell fractionation experiments, the transferase is essentially associated with membranes when it is recovered.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Dai D., Ishiguro E. E. murH, a new genetic locus in Escherichia coli involved in cell wall peptidoglycan biosynthesis. J Bacteriol. 1988 May;170(5):2197–2201. doi: 10.1128/jb.170.5.2197-2201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher G., Irwin C. A., Henson J. M., Fillingim C., Malone M. M., Walker J. R. Identification of the Escherichia coli cell division gene sep and organization of the cell division-cell envelope genes in the sep-mur-ftsA-envA cluster as determined with specialized transducing lambda bacteriophages. J Bacteriol. 1978 Jan;133(1):91–100. doi: 10.1128/jb.133.1.91-100.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flouret B., Mengin-Lecreulx D., van Heijenoort J. Reverse-phase high-pressure liquid chromatography of uridine diphosphate N-acetylmuramyl peptide precursors of bacterial cell wall peptidoglycan. Anal Biochem. 1981 Jun;114(1):59–63. doi: 10.1016/0003-2697(81)90451-6. [DOI] [PubMed] [Google Scholar]
- Geis A., Plapp R. Phospho-N-acetylmuramoyl-pentapeptide-transferase of Escherichia coli K12. Properties of the membrane-bound and the extracted and partially purified enzyme. Biochim Biophys Acta. 1978 Dec 8;527(2):414–424. doi: 10.1016/0005-2744(78)90355-8. [DOI] [PubMed] [Google Scholar]
- Glauner B. Separation and quantification of muropeptides with high-performance liquid chromatography. Anal Biochem. 1988 Aug 1;172(2):451–464. doi: 10.1016/0003-2697(88)90468-x. [DOI] [PubMed] [Google Scholar]
- Gondré B., Flouret B., van Heijenoort J. Release of D-alanyl-D-alanine from the precursor of the cell wall peptidoglycan by a peptidase of Escherichia coli K 12. Biochimie. 1973;55(6):685–691. doi: 10.1016/s0300-9084(73)80022-7. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Sato T., Wachi M., Jung H. K., Ishino F., Kobayashi Y., Matsuhashi M. Structural similarity among Escherichia coli FtsW and RodA proteins and Bacillus subtilis SpoVE protein, which function in cell division, cell elongation, and spore formation, respectively. J Bacteriol. 1989 Nov;171(11):6375–6378. doi: 10.1128/jb.171.11.6375-6378.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M., Wachi M., Ishino F., Matsuhashi M. Nucleotide sequence involving murD and an open reading frame ORF-Y spacing murF and ftsW in Escherichia coli. Nucleic Acids Res. 1990 Feb 25;18(4):1058–1058. doi: 10.1093/nar/18.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M., Wachi M., Jung H. K., Ishino F., Matsuhashi M. Nucleotide sequence involving murG and murC in the mra gene cluster region of Escherichia coli. Nucleic Acids Res. 1990 Jul 11;18(13):4014–4014. doi: 10.1093/nar/18.13.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M., Wachi M., Jung H. K., Ishino F., Matsuhashi M. The Escherichia coli mraY gene encoding UDP-N-acetylmuramoyl-pentapeptide: undecaprenyl-phosphate phospho-N-acetylmuramoyl-pentapeptide transferase. J Bacteriol. 1991 Feb;173(3):1021–1026. doi: 10.1128/jb.173.3.1021-1026.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E., Ramey W. D. Involvement of the relA gene product and feedback inhibition in the regulation of DUP-N-acetylmuramyl-peptide synthesis in Escherichia coli. J Bacteriol. 1978 Sep;135(3):766–774. doi: 10.1128/jb.135.3.766-774.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino F., Jung H. K., Ikeda M., Doi M., Wachi M., Matsuhashi M. New mutations fts-36, lts-33, and ftsW clustered in the mra region of the Escherichia coli chromosome induce thermosensitive cell growth and division. J Bacteriol. 1989 Oct;171(10):5523–5530. doi: 10.1128/jb.171.10.5523-5530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Kohlrausch U., Wientjes F. B., Höltje J. V. Determination of murein precursors during the cell cycle of Escherichia coli. J Gen Microbiol. 1989 Jun;135(6):1499–1506. doi: 10.1099/00221287-135-6-1499. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Leduc M., Fréhel C., Siegel E., Van Heijenoort J. Multilayered distribution of peptidoglycan in the periplasmic space of Escherichia coli. J Gen Microbiol. 1989 May;135(5):1243–1254. doi: 10.1099/00221287-135-5-1243. [DOI] [PubMed] [Google Scholar]
- Leplatois P., Danchin A. Vectors for high conditional expression of cloned genes. Biochimie. 1983 Jun;65(6):317–324. doi: 10.1016/s0300-9084(83)80153-9. [DOI] [PubMed] [Google Scholar]
- Lugtenberg E. J., de Haan P. G. A simple method for following the fate of alanine-containing components in murein synthesis in Escherichia coli. Antonie Van Leeuwenhoek. 1971;37(4):537–552. doi: 10.1007/BF02218524. [DOI] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutant of Escherichia coli K-12 with an impaired D-alanine:D-alanine ligase. J Bacteriol. 1973 Jan;113(1):96–104. doi: 10.1128/jb.113.1.96-104.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutants of Escherichia coli K-12 with low activities of the L-alanine adding enzyme and the D-alanyl-D-alanine adding enzyme. J Bacteriol. 1972 Apr;110(1):35–40. doi: 10.1128/jb.110.1.35-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutants of Escherichia coli K-12 with low activity of the diaminopimelic acid adding enzyme. J Bacteriol. 1972 Apr;110(1):41–46. doi: 10.1128/jb.110.1.41-46.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A., van Bellegem T. H. In vivo and in vitro action of new antibiotics interfering with the utilization of N-acetyl-glucosamine-N-acetyl-muramyl-pentapeptide. J Bacteriol. 1971 Oct;108(1):20–29. doi: 10.1128/jb.108.1.20-29.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. Regulation of cell division in E. coli. Trends Genet. 1990 Jan;6(1):22–25. doi: 10.1016/0168-9525(90)90045-8. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mengin-Lecreulx D., Flouret B., van Heijenoort J. Cytoplasmic steps of peptidoglycan synthesis in Escherichia coli. J Bacteriol. 1982 Sep;151(3):1109–1117. doi: 10.1128/jb.151.3.1109-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., Flouret B., van Heijenoort J. Pool levels of UDP N-acetylglucosamine and UDP N-acetylglucosamine-enolpyruvate in Escherichia coli and correlation with peptidoglycan synthesis. J Bacteriol. 1983 Jun;154(3):1284–1290. doi: 10.1128/jb.154.3.1284-1290.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., Michaud C., Richaud C., Blanot D., van Heijenoort J. Incorporation of LL-diaminopimelic acid into peptidoglycan of Escherichia coli mutants lacking diaminopimelate epimerase encoded by dapF. J Bacteriol. 1988 May;170(5):2031–2039. doi: 10.1128/jb.170.5.2031-2039.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., Parquet C., Desviat L. R., Plá J., Flouret B., Ayala J. A., van Heijenoort J. Organization of the murE-murG region of Escherichia coli: identification of the murD gene encoding the D-glutamic-acid-adding enzyme. J Bacteriol. 1989 Nov;171(11):6126–6134. doi: 10.1128/jb.171.11.6126-6134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., Siegel E., van Heijenoort J. Variations in UDP-N-acetylglucosamine and UDP-N-acetylmuramyl-pentapeptide pools in Escherichia coli after inhibition of protein synthesis. J Bacteriol. 1989 Jun;171(6):3282–3287. doi: 10.1128/jb.171.6.3282-3287.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., Texier L., van Heijenoort J. Nucleotide sequence of the cell-envelope murG gene of Escherichia coli. Nucleic Acids Res. 1990 May 11;18(9):2810–2810. doi: 10.1093/nar/18.9.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., van Heijenoort J. Effect of growth conditions on peptidoglycan content and cytoplasmic steps of its biosynthesis in Escherichia coli. J Bacteriol. 1985 Jul;163(1):208–212. doi: 10.1128/jb.163.1.208-212.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., van Heijenoort J. Nucleotide sequence of the murD gene encoding the UDP-MurNAc-L-Ala-D-Glu synthetase of Escherichia coli. Nucleic Acids Res. 1990 Jan 11;18(1):183–183. doi: 10.1093/nar/18.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud C., Blanot D., Flouret B., Van Heijenoort J. Partial purification and specificity studies of the D-glutamate-adding and D-alanyl-D-alanine-adding enzymes from Escherichia coli K12. Eur J Biochem. 1987 Aug 3;166(3):631–637. doi: 10.1111/j.1432-1033.1987.tb13560.x. [DOI] [PubMed] [Google Scholar]
- Michaud C., Parquet C., Flouret B., Blanot D., van Heijenoort J. Revised interpretation of the sequence containing the murE gene encoding the UDP-N-acetylmuramyl-tripeptide synthetase of Escherichia coli. Biochem J. 1990 Jul 1;269(1):277–278. doi: 10.1042/bj2690277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T., Matsuzawa H., Matsuhashi M., Sugino Y. Cell wall peptidoglycan mutants of Escherichia coli K-12: existence of two clusters of genes, mra and mrb, for cell wall peptidoglycan biosynthesis. J Bacteriol. 1972 Nov;112(2):950–958. doi: 10.1128/jb.112.2.950-958.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Maruyama I. N., Soma M., Kato J., Suzuki H., Horota Y. On the process of cellular division in Escherichia coli: nucleotide sequence of the gene for penicillin-binding protein 3. Mol Gen Genet. 1983;191(1):1–9. doi: 10.1007/BF00330881. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Takeda Y., Nishimura A., Suzuki H., Inouye M., Hirota Y. Synthetic ColE1 plasmids carrying genes for cell division in Escherichia coli. Plasmid. 1977 Nov;1(1):67–77. doi: 10.1016/0147-619x(77)90009-9. [DOI] [PubMed] [Google Scholar]
- Prats R., de Pedro M. A. Normal growth and division of Escherichia coli with a reduced amount of murein. J Bacteriol. 1989 Jul;171(7):3740–3745. doi: 10.1128/jb.171.7.3740-3745.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramey W. D., Ishiguro E. E. Site of inhibition of peptidoglycan biosynthesis during the stringent response in Escherichia coli. J Bacteriol. 1978 Jul;135(1):71–77. doi: 10.1128/jb.135.1.71-77.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond G. P., Lutkenhaus J. F., Donachie W. D. Identification of new genes in a cell envelope-cell division gene cluster of Escherichia coli: cell envelope gene murG. J Bacteriol. 1980 Oct;144(1):438–440. doi: 10.1128/jb.144.1.438-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Temperature-sensitive cell division mutants of Escherichia coli with thermolabile penicillin-binding proteins. J Bacteriol. 1977 Jul;131(1):293–305. doi: 10.1128/jb.131.1.293-305.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Nishimura A., Nishimura Y., Yamada M., Yasuda S., Suzuki H., Hirota Y. Synthetic ColE1 Plasmids carrying genes for penicillin-binding proteins in Escherichia coli. Plasmid. 1981 Jul;6(1):86–98. doi: 10.1016/0147-619x(81)90056-1. [DOI] [PubMed] [Google Scholar]
- Wientjes F. B., Pas E., Taschner P. E., Woldringh C. L. Kinetics of uptake and incorporation of meso-diaminopimelic acid in different Escherichia coli strains. J Bacteriol. 1985 Oct;164(1):331–337. doi: 10.1128/jb.164.1.331-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman H. J. A genetic map of several mutations affecting the mucopeptide layer of Escherichia coli. Genet Res. 1972 Aug;20(1):65–74. doi: 10.1017/s0016672300013598. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Venkateswaran P. S. Fosfomycin-resistant mutant of Escherichia coli. Ann N Y Acad Sci. 1974 May 10;235(0):587–592. doi: 10.1111/j.1749-6632.1974.tb43292.x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]