Abstract
Salmonella enteritidis was previously shown to produce fimbriae composed of 14,000-molecular-weight (Mr) fimbrin monomers (J. Feutrier, W. W. Kay, and T. J. Trust, J. Bacteriol. 168:221-227, 1986). Another distinct fimbrial structure, comprising 21,000-Mr fimbrin monomers, has now been identified. These fimbriae are simply designated as SEF 14 and SEF 21, respectively (for S. enteritidis fimbriae and the Mr [in thousands] of the fimbrin monomer). A simple method for the purification of both structures was developed by using the different biochemical properties of these fimbriae. SEF 21 remained intact after being boiled in sodium dodecyl sulfate but readily dissociated into subunits of 21,000 Mr at pH 2.2. The overall amino acid composition and the N-terminal amino acid sequence of the SEF 21 fimbrin were distinct from those of SEF 14 but were virtually identical to the predicted sequence for type 1 fimbrin of Salmonella typhimurium. Immunoelectron microscopy of S. enteritidis clearly revealed fimbrial structures that reacted with immune serum specific to the 21,000-Mr fimbrin. Immune sera raised against this subunit were cross-reactive with type 1 fimbrins found in whole-cell lysates of S. typhimurium, Salmonella illinois, and Salmonella cubana. However, there was no cross-reaction with Escherichia coli type 1 fimbriae or with other fimbrins produced by S. enteritidis. Under certain growth conditions, S. enteritidis produced both SEF 14 and SEF 21. However, when S. enteritidis was grown at 30 degrees C or lower, only the 21,000-Mr SEF 21 fimbrin could be detected. There was a direct correlation between mannose-sensitive hemagglutination and the presence of SEF 21.
Full text
PDF




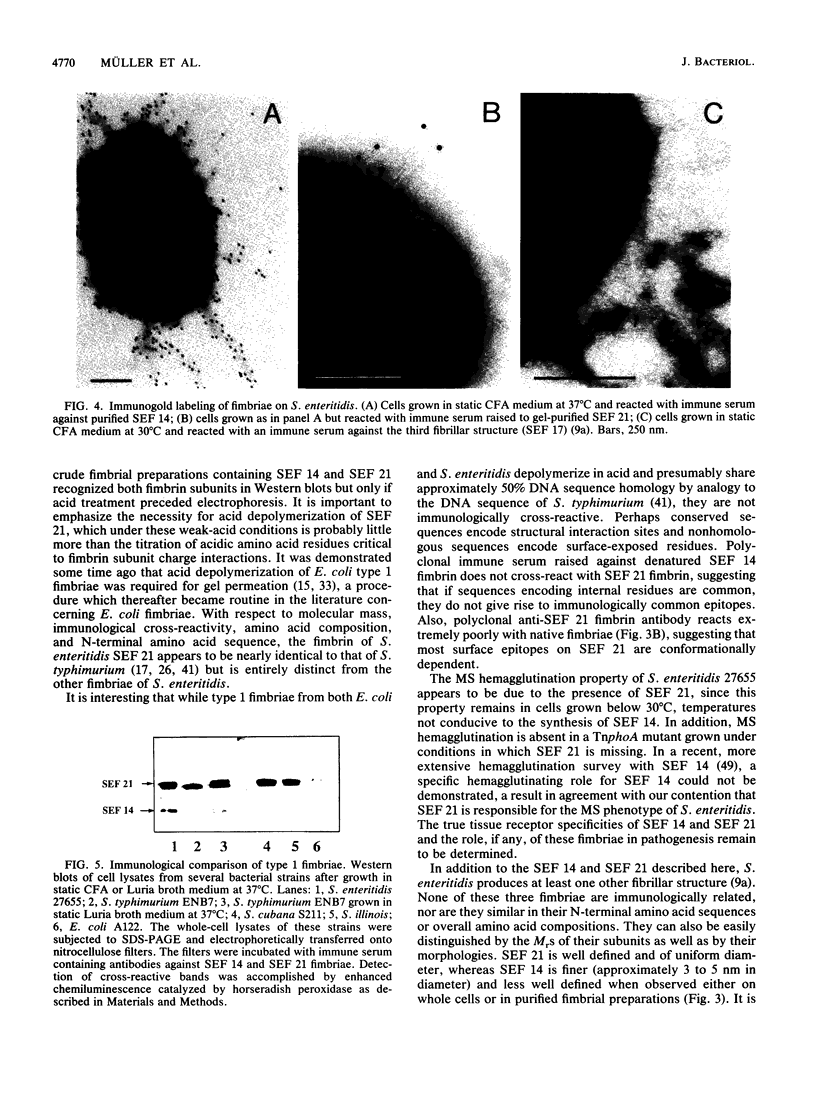


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson M., Medalia O., Schori L., Mirelman D., Sharon N., Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J Infect Dis. 1979 Mar;139(3):329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- Bloch C. A., Orndorff P. E. Impaired colonization by and full invasiveness of Escherichia coli K1 bearing a site-directed mutation in the type 1 pilin gene. Infect Immun. 1990 Jan;58(1):275–278. doi: 10.1128/iai.58.1.275-278.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. Mechanisms of bacterial virulence. Annu Rev Microbiol. 1985;39:21–50. doi: 10.1146/annurev.mi.39.100185.000321. [DOI] [PubMed] [Google Scholar]
- Buchanan K., Falkow S., Hull R. A., Hull S. I. Frequency among Enterobacteriaceae of the DNA sequences encoding type 1 pili. J Bacteriol. 1985 May;162(2):799–803. doi: 10.1128/jb.162.2.799-803.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Carsiotis M., Weinstein D. L., Karch H., Holder I. A., O'Brien A. D. Flagella of Salmonella typhimurium are a virulence factor in infected C57BL/6J mice. Infect Immun. 1984 Dec;46(3):814–818. doi: 10.1128/iai.46.3.814-818.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg S., Gerlach G. F. Enterobacterial fimbriae. J Bacteriol. 1987 Mar;169(3):934–938. doi: 10.1128/jb.169.3.934-938.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg S., Purcell B. K., Pruckler J. Characterization of genes encoding type 1 fimbriae of Klebsiella pneumoniae, Salmonella typhimurium, and Serratia marcescens. Infect Immun. 1987 Feb;55(2):281–287. doi: 10.1128/iai.55.2.281-287.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. L., Tauxe R. V. Drug-resistant Salmonella in the United States: an epidemiologic perspective. Science. 1986 Nov 21;234(4779):964–969. doi: 10.1126/science.3535069. [DOI] [PubMed] [Google Scholar]
- Collinson S. K., Emödy L., Müller K. H., Trust T. J., Kay W. W. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J Bacteriol. 1991 Aug;173(15):4773–4781. doi: 10.1128/jb.173.15.4773-4781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke E. M. Epidemiology of foodborne illness: UK. Lancet. 1990 Sep 29;336(8718):790–793. doi: 10.1016/0140-6736(90)93251-j. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Kelly S. M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987 Dec;55(12):3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig P., Sastry P. A., Hodges R. S., Lee K. K., Paranchych W., Irvin R. T. Inhibition of pilus-mediated adhesion of Pseudomonas aeruginosa to human buccal epithelial cells by monoclonal antibodies directed against pili. Infect Immun. 1990 Jan;58(1):124–130. doi: 10.1128/iai.58.1.124-130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Darekar M. R., Wheater D. W. Fimbriae and infectivity in Salmonella typhimurium. J Med Microbiol. 1976 Nov;9(4):459–473. doi: 10.1099/00222615-9-4-459. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., Silverblatt F. J., Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981 Oct;148(1):308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977 Nov;18(2):330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feutrier J., Kay W. W., Trust T. J. Cloning and expression of a Salmonella enteritidis fimbrin gene in Escherichia coli. J Bacteriol. 1988 Sep;170(9):4216–4222. doi: 10.1128/jb.170.9.4216-4222.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feutrier J., Kay W. W., Trust T. J. Purification and characterization of fimbriae from Salmonella enteritidis. J Bacteriol. 1986 Oct;168(1):221–227. doi: 10.1128/jb.168.1.221-227.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Heffron F., Falkow S. Epithelial cell surfaces induce Salmonella proteins required for bacterial adherence and invasion. Science. 1989 Feb 17;243(4893):940–943. doi: 10.1126/science.2919285. [DOI] [PubMed] [Google Scholar]
- Groisman E. A., Saier M. H., Jr Salmonella virulence: new clues to intramacrophage survival. Trends Biochem Sci. 1990 Jan;15(1):30–33. doi: 10.1016/0968-0004(90)90128-x. [DOI] [PubMed] [Google Scholar]
- Hohmann A. W., Schmidt G., Rowley D. Intestinal colonization and virulence of Salmonella in mice. Infect Immun. 1978 Dec;22(3):763–770. doi: 10.1128/iai.22.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahi T., Abe Y., Nakao M., Imada A., Tsuchiya K. Role of type 1 fimbriae in the pathogenesis of ascending urinary tract infection induced by escherichia coli in mice. Infect Immun. 1983 Mar;39(3):1307–1315. doi: 10.1128/iai.39.3.1307-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Richardson L. A. The attachment to, and invasion of HeLa cells by Salmonella typhimurium: the contribution of mannose-sensitive and mannose-resistant haemagglutinating activities. J Gen Microbiol. 1981 Dec;127(2):361–370. doi: 10.1099/00221287-127-2-361. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Lounatmaa K., Ranta H., Kuusi N. Characterization of type 1 pili of Salmonella typhimurium LT2. J Bacteriol. 1980 Nov;144(2):800–805. doi: 10.1128/jb.144.2.800-805.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGendre N., Matsudaira P. Direct protein microsequencing from Immobilon-P Transfer Membrane. Biotechniques. 1988 Feb;6(2):154–159. [PubMed] [Google Scholar]
- Lindquist B. L., Lebenthal E., Lee P. C., Stinson M. W., Merrick J. M. Adherence of Salmonella typhimurium to small-intestinal enterocytes of the rat. Infect Immun. 1987 Dec;55(12):3044–3050. doi: 10.1128/iai.55.12.3044-3050.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Lindberg F. P., Båga M., Normark S. Globoside-specific adhesins of uropathogenic Escherichia coli are encoded by similar trans-complementable gene clusters. J Bacteriol. 1985 Jun;162(3):1293–1301. doi: 10.1128/jb.162.3.1293-1301.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Marklund B. I., Strömberg N., Lindberg F., Karlsson K. A., Normark S. Uropathogenic Escherichia coli can express serologically identical pili of different receptor binding specificities. Mol Microbiol. 1988 Mar;2(2):255–263. doi: 10.1111/j.1365-2958.1988.tb00027.x. [DOI] [PubMed] [Google Scholar]
- McCormick B. A., Franklin D. P., Laux D. C., Cohen P. S. Type 1 pili are not necessary for colonization of the streptomycin-treated mouse large intestine by type 1-piliated Escherichia coli F-18 and E. coli K-12. Infect Immun. 1989 Oct;57(10):3022–3029. doi: 10.1128/iai.57.10.3022-3029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Structure of common pili from Escherichia coli. J Bacteriol. 1979 Jun;138(3):969–975. doi: 10.1128/jb.138.3.969-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Miller S. I., Kukral A. M., Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K. H., Trust T. J., Kay W. W. Fimbriation genes of Salmonella enteritidis. J Bacteriol. 1989 Sep;171(9):4648–4654. doi: 10.1128/jb.171.9.4648-4654.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki B., Rhen M., Väisänen-Rhen V., Pere A., Korhonen T. K. Immunofluorescence study of fimbrial phase variation in Escherichia coli KS71. J Bacteriol. 1984 Nov;160(2):691–695. doi: 10.1128/jb.160.2.691-695.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranchych W., Frost L. S. The physiology and biochemistry of pili. Adv Microb Physiol. 1988;29:53–114. doi: 10.1016/s0065-2911(08)60346-x. [DOI] [PubMed] [Google Scholar]
- Parkkinen J., Ristimäki A., Westerlund B. Binding of Escherichia coli S fimbriae to cultured human endothelial cells. Infect Immun. 1989 Jul;57(7):2256–2259. doi: 10.1128/iai.57.7.2256-2259.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popiel I., Turnbull P. C. Passage of Salmonella enteritidis and Salmonella thompson through chick ileocecal mucosa. Infect Immun. 1985 Mar;47(3):786–792. doi: 10.1128/iai.47.3.786-792.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell B. K., Pruckler J., Clegg S. Nucleotide sequences of the genes encoding type 1 fimbrial subunits of Klebsiella pneumoniae and Salmonella typhimurium. J Bacteriol. 1987 Dec;169(12):5831–5834. doi: 10.1128/jb.169.12.5831-5834.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G., Sobel J. D. Bacterial adherence in the pathogenesis of urinary tract infection: a review. Rev Infect Dis. 1987 May-Jun;9(3):470–487. doi: 10.1093/clinids/9.3.470. [DOI] [PubMed] [Google Scholar]
- Rodrigue D. C., Tauxe R. V., Rowe B. International increase in Salmonella enteritidis: a new pandemic? Epidemiol Infect. 1990 Aug;105(1):21–27. doi: 10.1017/s0950268800047609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareneva T., Holthöfer H., Korhonen T. K. Tissue-binding affinity of Proteus mirabilis fimbriae in the human urinary tract. Infect Immun. 1990 Oct;58(10):3330–3336. doi: 10.1128/iai.58.10.3330-3336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Ofek I. Influence of pili on the virulence of Proteus mirabilis in experimental hematogenous pyelonephritis. J Infect Dis. 1978 Nov;138(5):664–667. doi: 10.1093/infdis/138.5.664. [DOI] [PubMed] [Google Scholar]
- Thorns C. J., Sojka M. G., Chasey D. Detection of a novel fimbrial structure on the surface of Salmonella enteritidis by using a monoclonal antibody. J Clin Microbiol. 1990 Nov;28(11):2409–2414. doi: 10.1128/jcm.28.11.2409-2414.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe G. H., Kricka L. J. Enhanced chemiluminescent reactions catalyzed by horseradish peroxidase. Methods Enzymol. 1986;133:331–353. doi: 10.1016/0076-6879(86)33078-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Ariyoshi A., Amako K. Fimbria-mediated adherence of Serratia marcescens strain US5 to human urinary bladder surface. Microbiol Immunol. 1985;29(7):677–681. doi: 10.1111/j.1348-0421.1985.tb00871.x. [DOI] [PubMed] [Google Scholar]







