Abstract
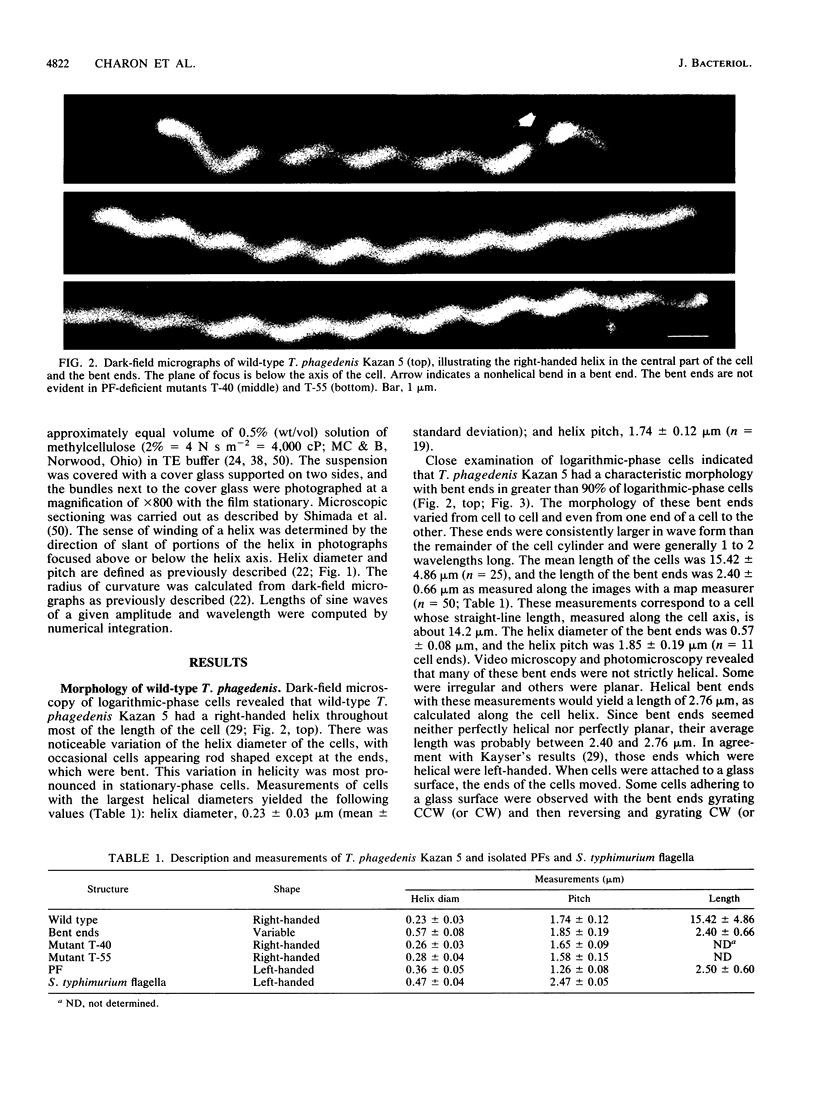
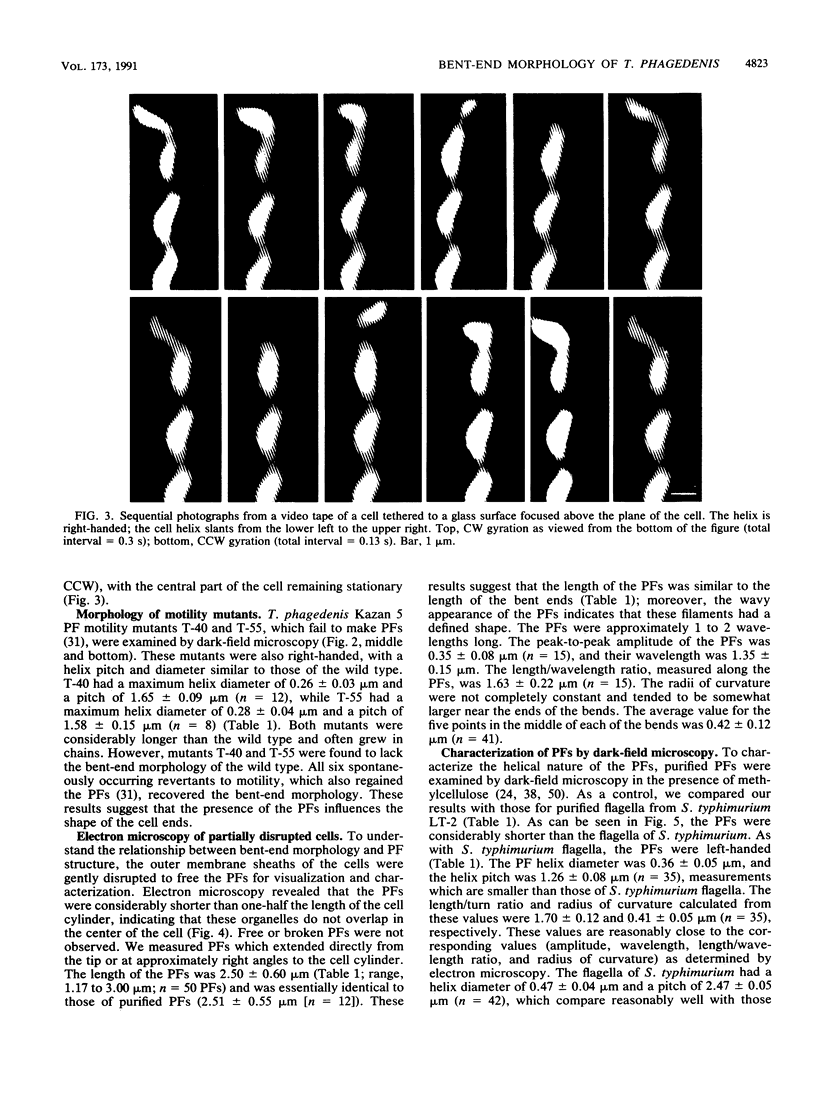
Treponema phagedenis Kazan 5 is a spirochete with multiple periplasmic flagella attached near each end of the cell cylinder. Dark-field microscopy revealed that most of the cell is right-handed (helix diameter, 0.23 micron; helix pitch, 1.74 microns), and the ends appear bent. These ends could move and gyrate while the central part of the cell remained stationary. The present study examines the basis for the bent-end characteristic. Motility mutants deficient in periplasmic flagella were found to lack the bent ends, and spontaneous revertants to motility regained the periplasmic flagella and bent-end characteristic. The length of the bent ends (2.40 microns) was found to be similar to the length of the periplasmic flagella as determined by electron microscopy (2.50 microns). The helix diameter of the bent ends was 0.57 micron, and the helix pitch of the bent ends was 1.85 microns. The periplasmic flagella were short relative to the length of the cells (15 microns) and, in contrast to the reports of others, did not overlap in the center of the cell. Similar results were found with T. phagedenis Reiter. The results taken together indicate that there is a causal relationship between the bent-end morphology and the presence of short periplasmic flagella. We report the first three-dimensional description of spirochete periplasmic flagella. Dark-field microscopy of purified periplasmic flagella revealed that these organelles were left-handed (helix diameter, 0.36 microns; helix pitch, 1.26 microns) and only 1 to 2 wavelengths long. Because of a right-handed cell cylinder and left-handed periplasmic flagella along with bent ends having helix diameters greater than those of either the cell cylinder or periplasmic flagella, we conclude that there is a complex interaction of the periplasmic flagella and the cell cylinder to form the bent ends. The results are discussed with respect to a possible mechanism of T. phagedenis motility.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa S. I., Dean G. E., Jones C. J., Macnab R. M., Yamaguchi S. Purification and characterization of the flagellar hook-basal body complex of Salmonella typhimurium. J Bacteriol. 1985 Mar;161(3):836–849. doi: 10.1128/jb.161.3.836-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADFIELD J. R. G., CATER D. B. Electron-microscopic evidence on the structure of spirochaetes. Nature. 1952 Jun 7;169(4310):944–946. doi: 10.1038/169944a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C. How spirochetes may swim. J Theor Biol. 1976 Feb;56(2):269–273. doi: 10.1016/s0022-5193(76)80074-4. [DOI] [PubMed] [Google Scholar]
- Bharier M., Allis D. Purification and characterization of axial filaments from Treponema phagedenis biotype reiterii (the Reiter treponeme). J Bacteriol. 1974 Dec;120(3):1434–1442. doi: 10.1128/jb.120.3.1434-1442.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch-Andersen A., Hovind Hougen K., Borg-Petersen C. Electron microscopy of Leptospira. 1. Leptospira strain Pomona. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Dec;81(6):665–676. doi: 10.1111/j.1699-0463.1973.tb02258.x. [DOI] [PubMed] [Google Scholar]
- Blair D. F. The bacterial flagellar motor. Semin Cell Biol. 1990 Apr;1(2):75–85. [PubMed] [Google Scholar]
- Blanco D. R., Champion C. I., Miller J. N., Lovett M. A. Antigenic and structural characterization of Treponema pallidum (Nichols strain) endoflagella. Infect Immun. 1988 Jan;56(1):168–175. doi: 10.1128/iai.56.1.168-175.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahamsha B., Greenberg E. P. Biochemical and cytological analysis of the complex periplasmic flagella from Spirochaeta aurantia. J Bacteriol. 1988 Sep;170(9):4023–4032. doi: 10.1128/jb.170.9.4023-4032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley D. B., Charon N. W. Axial filament involvement in the motility of Leptospira interrogans. J Bacteriol. 1979 Mar;137(3):1406–1412. doi: 10.1128/jb.137.3.1406-1412.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIANSEN A. H. STUDIES ON THE ANTIGENIC STRUCTURE OF T. PALLIDUM. 4. COMPARISON BETWEEN THE CULTIVABLE STRAINS T. REITER AND T. KAZAN II, APPLYING AGAR GEL DIFFUSION TECHNIQUE AND CROSS ABSORPTION EXPERIMENTS. Acta Pathol Microbiol Scand. 1964;60:123–130. doi: 10.1111/apm.1964.60.1.123. [DOI] [PubMed] [Google Scholar]
- Canale-Parola E. Motility and chemotaxis of spirochetes. Annu Rev Microbiol. 1978;32:69–99. doi: 10.1146/annurev.mi.32.100178.000441. [DOI] [PubMed] [Google Scholar]
- Champion C. I., Miller J. N., Lovett M. A., Blanco D. R. Cloning, sequencing, and expression of two class B endoflagellar genes of Treponema pallidum subsp. pallidum encoding the 34.5- and 31.0-kilodalton proteins. Infect Immun. 1990 Jun;58(6):1697–1704. doi: 10.1128/iai.58.6.1697-1704.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Daughtry G. R., McCuskey R. S., Franz G. N. Microcinematographic analysis of tethered Leptospira illini. J Bacteriol. 1984 Dec;160(3):1067–1073. doi: 10.1128/jb.160.3.1067-1073.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Lawrence C. W., O'Brien S. Movement of antibody-coated latex beads attached to the spirochete Leptospira interrogans. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7166–7170. doi: 10.1073/pnas.78.11.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne A., Bailey M. J., Penn C. W. Analysis of sheath and core structures of the axial filament of Treponema pallidum. J Gen Microbiol. 1987 Jun;133(6):1397–1407. doi: 10.1099/00221287-133-6-1397. [DOI] [PubMed] [Google Scholar]
- Cockayne A., Sanger R., Ivic A., Strugnell R. A., MacDougall J. H., Russell R. R., Penn C. W. Antigenic and structural analysis of Treponema denticola. J Gen Microbiol. 1989 Dec;135(12):3209–3218. doi: 10.1099/00221287-135-12-3209. [DOI] [PubMed] [Google Scholar]
- Cox C. D. Shape of Treponema pallidum. J Bacteriol. 1972 Feb;109(2):943–944. doi: 10.1128/jb.109.2.943-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosnaugh K., Greenberg E. P. Motility and chemotaxis of Spirochaeta aurantia: computer-assisted motion analysis. J Bacteriol. 1988 Apr;170(4):1768–1774. doi: 10.1128/jb.170.4.1768-1774.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S. F., Charon N. W. Motility of the spirochete Leptospira. Cell Motil Cytoskeleton. 1988;9(2):101–110. doi: 10.1002/cm.970090202. [DOI] [PubMed] [Google Scholar]
- Goldstein S. F., Charon N. W. Multiple-exposure photographic analysis of a motile spirochete. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4895–4899. doi: 10.1073/pnas.87.13.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978 Mar;42(1):114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotani H. Light microscope study of mixed helices in reconstituted Salmonella flagella. J Mol Biol. 1976 Sep 5;106(1):151–166. doi: 10.1016/0022-2836(76)90305-3. [DOI] [PubMed] [Google Scholar]
- Hougen K. H., Birch-Andersen A. Electron microscopy of endoflagella and microtubules in Treponema reiter. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(1):37–50. doi: 10.1111/j.1699-0463.1971.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Hougen K. H. The ultrastructure of cultivable treponemes. 1. Treponema phagedenis, Treponema vincentii and Treponema refringens. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):329–344. [PubMed] [Google Scholar]
- Hovind-Hougen K. Determination by means of electron microscopy of morphological criteria of value for classification of some spirochetes, in particular treponemes. Acta Pathol Microbiol Scand Suppl. 1976;(255):1–41. [PubMed] [Google Scholar]
- Iino T., Enomoto M. Genetical studies of non-flagellate mutants of Salmonella. J Gen Microbiol. 1966 Jun;43(3):315–327. doi: 10.1099/00221287-43-3-315. [DOI] [PubMed] [Google Scholar]
- Kayser A., Adrian M. Les spirochètes: sens de l'enroulement. Ann Microbiol (Paris) 1978 Apr;129(3):351–360. [PubMed] [Google Scholar]
- Limberger R. J., Charon N. W. Antiserum to the 33,000-dalton periplasmic-flagellum protein of "Treponema phagedenis" reacts with other treponemes and Spirochaeta aurantia. J Bacteriol. 1986 Nov;168(2):1030–1032. doi: 10.1128/jb.168.2.1030-1032.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberger R. J., Charon N. W. Treponema phagedenis has at least two proteins residing together on its periplasmic flagella. J Bacteriol. 1986 Apr;166(1):105–112. doi: 10.1128/jb.166.1.105-112.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Aizawa S. Bacterial motility and the bacterial flagellar motor. Annu Rev Biophys Bioeng. 1984;13:51–83. doi: 10.1146/annurev.bb.13.060184.000411. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Ornston M. K. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J Mol Biol. 1977 May 5;112(1):1–30. doi: 10.1016/s0022-2836(77)80153-8. [DOI] [PubMed] [Google Scholar]
- Miao R., Fieldsteel A. H. Genetics of Treponema: relationship between Treponema pallidum and five cultivable treponemes. J Bacteriol. 1978 Jan;133(1):101–107. doi: 10.1128/jb.133.1.101-107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki-Noumura T. Dark-field microscopic study of microtubules in solution. Int Rev Cytol. 1990;122:65–104. doi: 10.1016/s0074-7696(08)61206-1. [DOI] [PubMed] [Google Scholar]
- Nauman R. K., Holt S. C., Cox C. D. Purification, ultrastructure, and composition of axial filaments from Leptospira. J Bacteriol. 1969 Apr;98(1):264–280. doi: 10.1128/jb.98.1.264-280.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S. J., Charon N. W., Cook R. G., Fuentes M. D., Limberger R. J. Antigenic relatedness and N-terminal sequence homology define two classes of periplasmic flagellar proteins of Treponema pallidum subsp. pallidum and Treponema phagedenis. J Bacteriol. 1988 Sep;170(9):4072–4082. doi: 10.1128/jb.170.9.4072-4082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallesen L., Hindersson P. Cloning and sequencing of a Treponema pallidum gene encoding a 31.3-kilodalton endoflagellar subunit (FlaB2). Infect Immun. 1989 Jul;57(7):2166–2172. doi: 10.1128/iai.57.7.2166-2172.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parales J., Jr, Greenberg E. P. N-terminal amino acid sequences and amino acid compositions of the Spirochaeta aurantia flagellar filament polypeptides. J Bacteriol. 1991 Feb;173(3):1357–1359. doi: 10.1128/jb.173.3.1357-1359.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C. S., Pedersen N. S., Axelsen N. H. A simple method for the isolation of flagella from Treponema Reiter. Acta Pathol Microbiol Scand C. 1981 Dec;89(6):379–385. doi: 10.1111/j.1699-0463.1981.tb02716.x. [DOI] [PubMed] [Google Scholar]
- RYTER A., PILLOT J. [Electron microscope study of the external and internal structure of the Reiter treponema]. Ann Inst Pasteur (Paris) 1963 Apr;104:496–501. [PubMed] [Google Scholar]
- Radolf J. D., Blanco D. R., Miller J. N., Lovett M. A. Antigenic interrelationship between endoflagella of Treponema phagedenis biotype Reiter and Treponema pallidum (Nichols): molecular characterization of endoflagellar proteins. Infect Immun. 1986 Dec;54(3):626–634. doi: 10.1128/iai.54.3.626-634.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Kamiya R., Asakura S. Left-handed to right-handed helix conversion in Salmonella flagella. Nature. 1975 Mar 27;254(5498):332–334. doi: 10.1038/254332a0. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974 May 3;249(452):73–74. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]
- Strugnell R., Cockayne A., Penn C. W. Molecular and antigenic analysis of treponemes. Crit Rev Microbiol. 1990;17(4):231–250. doi: 10.3109/10408419009105727. [DOI] [PubMed] [Google Scholar]
- Sykes J. A., Miller J. N. Ultrastructural studies of treponemes: location of axial filaments and some dimensions of Treponema pallidum (Nichols strain), Treponema denticola, and Treponema reiteri. Infect Immun. 1973 Jan;7(1):100–110. doi: 10.1128/iai.7.1.100-110.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]






