Abstract
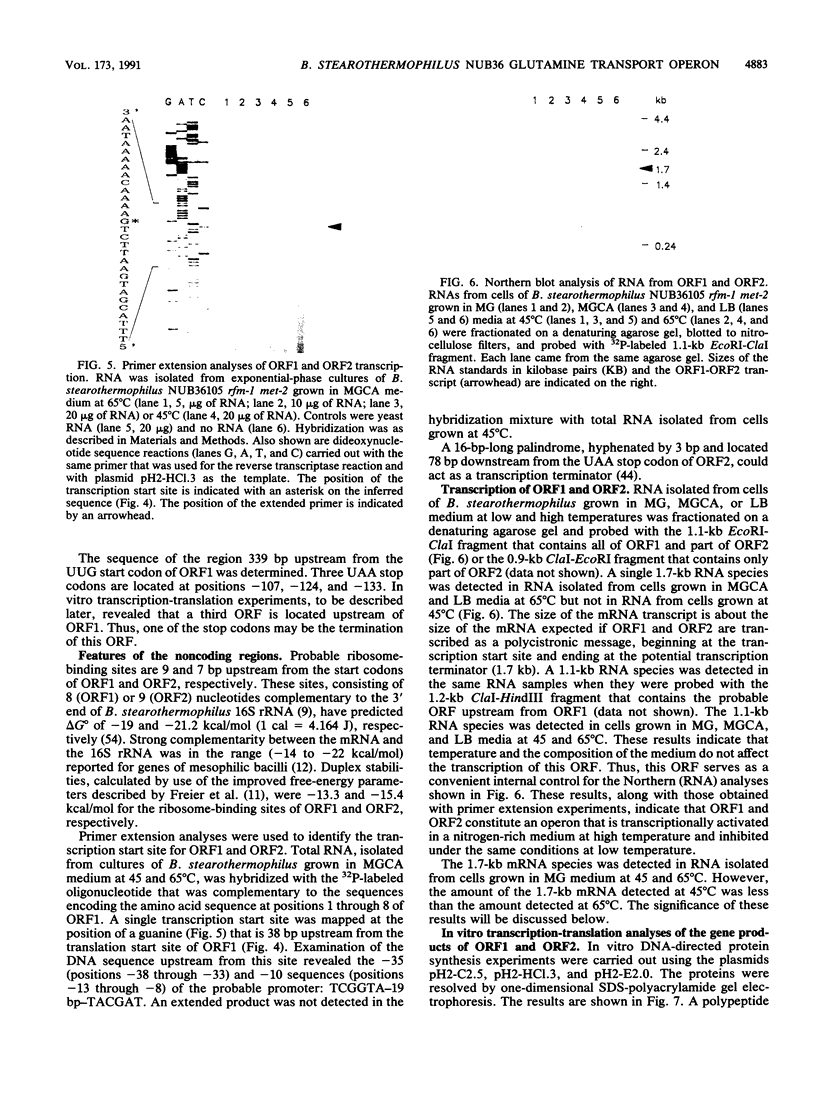
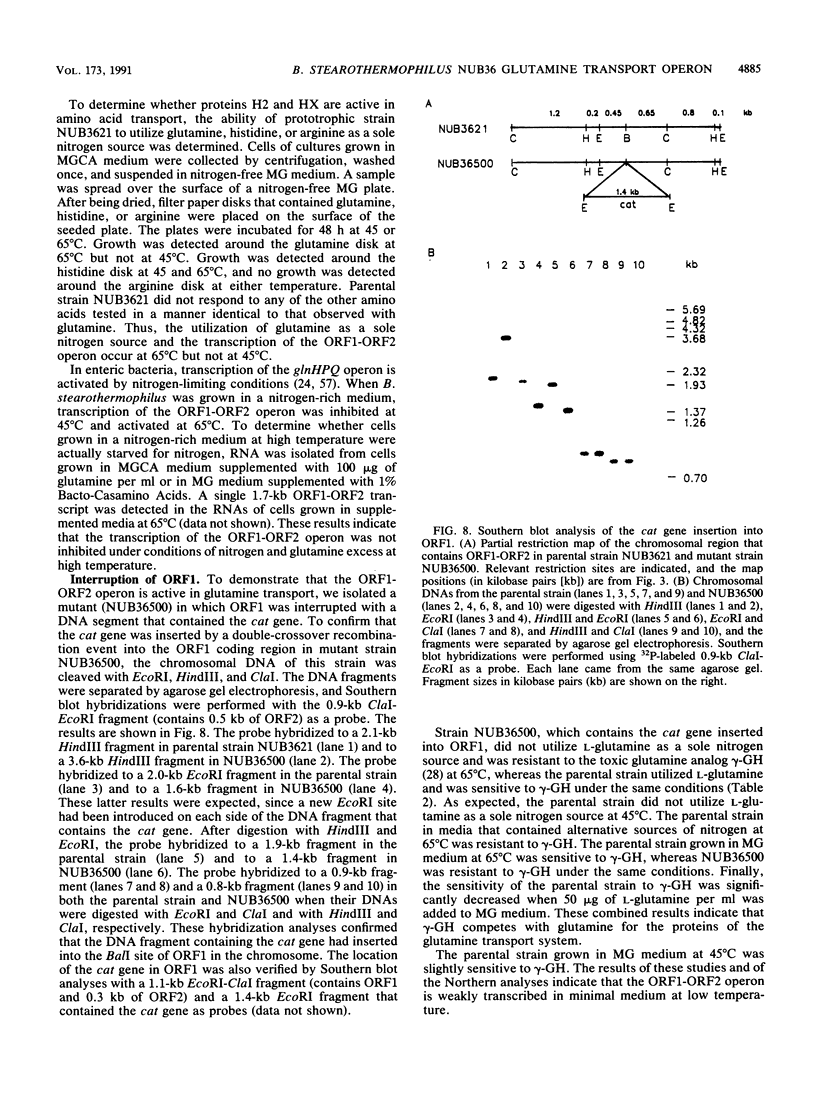
We cloned and sequenced a fragment of the Bacillus stearothermophilus NUB36 chromosome that contains two open reading frames (ORFs) whose products were detected only in cells of cultures grown in complex medium at high temperature. The nucleotide sequence of the two ORFs exhibited significant identity to the sequence of the glnQ and glnH loci of the glutamine transport system in enteric bacteria. In addition, growth response to glutamine, sensitivity to the toxic glutamine analog gamma-L-glutamylhydrazide, and glutamine transport assays with parental strain NUB3621 and mutant strain NUB36500, in which the ORF1 coding segment in the chromosome was interrupted with the cat gene, demonstrated that glnQ and glnH encode proteins that are active in the glutamine transport system in B. stearothermophilus. The inferred promoter for the glnQH operon exhibited a low homology to the -35 and -10 regions of the consensus promoter sequences of Bacillus subtilis and Escherichia coli genes. In addition, the inferred promoter for the glnQH operon also exhibited a low homology with the consensus promoter sequence deduced from the sequences of the promoters of nine different genes from B. stearothermophilus. Transcription of the glnQH operon was activated in a nitrogen-rich medium at high temperature and inhibited under the same conditions at low temperature. Transcription of the glnQH operon was partially activated in a nitrogen-poor medium at low temperature. The region upstream from glnQ contains sequences that have a low homology with the nitrogen regulator I-binding sequences and the nitrogen-regulated promoters of enteric bacteria. The effect of temperature on the regulation of the glnQH operon is discussed.
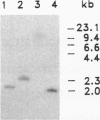
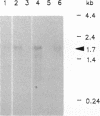
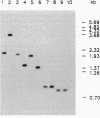
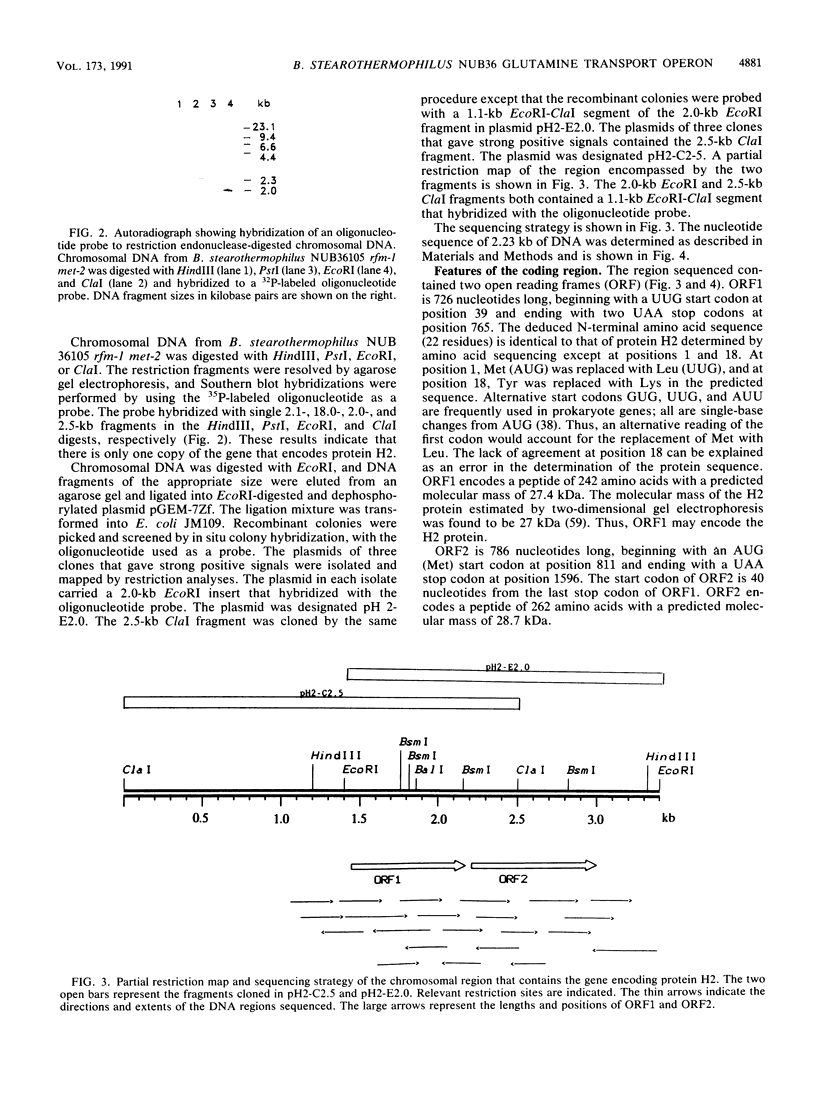
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barstow D. A., Sharman A. F., Atkinson T., Minton N. P. Cloning and complete nucleotide sequence of the Bacillus stearothermophilus tryptophanyl tRNA synthetase gene. Gene. 1986;46(1):37–45. doi: 10.1016/0378-1119(86)90164-2. [DOI] [PubMed] [Google Scholar]
- Bott K. F., Wilson G. A. Development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1967 Sep;94(3):562–570. doi: 10.1128/jb.94.3.562-570.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brush D., Dodgson J. B., Choi O. R., Stevens P. W., Engel J. D. Replacement variant histone genes contain intervening sequences. Mol Cell Biol. 1985 Jun;5(6):1307–1317. doi: 10.1128/mcb.5.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. F., Wojcik S. F., Welker N. E. Genetic analysis of Bacillus stearothermophilus by protoplast fusion. J Bacteriol. 1986 Mar;165(3):994–1001. doi: 10.1128/jb.165.3.994-1001.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultate T. P., Sundaram T. K. Energetics of Bacillus stearothermophilus growth: molar growth yield and temperature effects on growth efficiency. J Bacteriol. 1975 Jan;121(1):55–64. doi: 10.1128/jb.121.1.55-64.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi R. H., Wang L. F. Multiple procaryotic ribonucleic acid polymerase sigma factors. Microbiol Rev. 1986 Sep;50(3):227–243. doi: 10.1128/mr.50.3.227-243.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douthwaite S., Christensen A., Garrett R. A. Higher order structure in the 3'-minor domain of small subunit ribosomal RNAs from a gram negative bacterium, a gram positive bacterium and a eukaryote. J Mol Biol. 1983 Sep 5;169(1):249–279. doi: 10.1016/s0022-2836(83)80183-1. [DOI] [PubMed] [Google Scholar]
- Duvall E. J., Williams D. M., Mongkolsuk S., Lovett P. S. Regulatory regions that control expression of two chloramphenicol-inducible cat genes cloned in Bacillus subtilis. J Bacteriol. 1984 Jun;158(3):784–790. doi: 10.1128/jb.158.3.784-790.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Ames G. F. Two periplasmic transport proteins which interact with a common membrane receptor show extensive homology: complete nucleotide sequences. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6038–6042. doi: 10.1073/pnas.78.10.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Fukazawa T., Negoro S., Okada H. Structure of a beta-galactosidase gene of Bacillus stearothermophilus. J Bacteriol. 1986 Jun;166(3):722–727. doi: 10.1128/jb.166.3.722-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung L., Jost R., Stoll E., Zuber H. Metabolic differences in Bacillus stearothermophilus grown at 55 degrees C and 37 degrees C. Arch Mikrobiol. 1974 Feb 1;95(2):125–138. [PubMed] [Google Scholar]
- Kubo M., Imanaka T. Cloning and nucleotide sequence of the highly thermostable neutral protease gene from Bacillus stearothermophilus. J Gen Microbiol. 1988 Jul;134(7):1883–1892. doi: 10.1099/00221287-134-7-1883. [DOI] [PubMed] [Google Scholar]
- Kuriki T., Imanaka T. Nucleotide sequence of the neopullulanase gene from Bacillus stearothermophilus. J Gen Microbiol. 1989 Jun;135(6):1521–1528. doi: 10.1099/00221287-135-6-1521. [DOI] [PubMed] [Google Scholar]
- Kustu S. G., McFarland N. C., Hui S. P., Esmon B., Ames G. F. Nitrogen control of Salmonella typhimurium: co-regulation of synthesis of glutamine synthetase and amino acid transport systems. J Bacteriol. 1979 Apr;138(1):218–234. doi: 10.1128/jb.138.1.218-234.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Loprasert S., Negoro S., Okada H. Cloning, nucleotide sequence, and expression in Escherichia coli of the Bacillus stearothermophilus peroxidase gene (perA). J Bacteriol. 1989 Sep;171(9):4871–4875. doi: 10.1128/jb.171.9.4871-4875.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P. S., Hong J. S. Genetics of the glutamine transport system in Escherichia coli. J Bacteriol. 1981 Sep;147(3):805–819. doi: 10.1128/jb.147.3.805-819.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima R., Imanaka T., Aiba S. Nucleotide sequence of the Bacillus stearothermophilus alpha-amylase gene. J Bacteriol. 1985 Jul;163(1):401–406. doi: 10.1128/jb.163.1.401-406.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohno T., Saito T., Hong J. S. Cloning and complete nucleotide sequence of the Escherichia coli glutamine permease operon (glnHPQ). Mol Gen Genet. 1986 Nov;205(2):260–269. doi: 10.1007/BF00430437. [DOI] [PubMed] [Google Scholar]
- Nohno T., Saito T. Two transcriptional start sites found in the promoter region of Escherichia coli glutamine permease operon, glnHPQ. Nucleic Acids Res. 1987 Mar 25;15(6):2777–2777. doi: 10.1093/nar/15.6.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Parker J. Errors and alternatives in reading the universal genetic code. Microbiol Rev. 1989 Sep;53(3):273–298. doi: 10.1128/mr.53.3.273-298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Epstein I., Grossowicz N. Temperature-induced metabolic alterations in a thermophilic bacillus. Eur J Biochem. 1977 Aug 1;77(3):463–470. doi: 10.1111/j.1432-1033.1977.tb11687.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Werner P. K., Müller M. Insertion of proteins into bacterial membranes: mechanism, characteristics, and comparisons with the eucaryotic process. Microbiol Rev. 1989 Sep;53(3):333–366. doi: 10.1128/mr.53.3.333-366.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier H. J., Brown S. W., Hirschi K. D., Nomellini J. F., Sonenshein A. L. Regulation of Bacillus subtilis glutamine synthetase gene expression by the product of the glnR gene. J Mol Biol. 1989 Nov 5;210(1):51–63. doi: 10.1016/0022-2836(89)90290-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sullivan M. A., Yasbin R. E., Young F. E. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene. 1984 Jul-Aug;29(1-2):21–26. doi: 10.1016/0378-1119(84)90161-6. [DOI] [PubMed] [Google Scholar]
- Takagi M., Imanaka T., Aiba S. Nucleotide sequence and promoter region for the neutral protease gene from Bacillus stearothermophilus. J Bacteriol. 1985 Sep;163(3):824–831. doi: 10.1128/jb.163.3.824-831.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanizawa K., Ohshima A., Scheidegger A., Inagaki K., Tanaka H., Soda K. Thermostable alanine racemase from Bacillus stearothermophilus: DNA and protein sequence determination and secondary structure prediction. Biochemistry. 1988 Feb 23;27(4):1311–1316. doi: 10.1021/bi00404a033. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Vallier H., Welker N. E. Genetic map of the Bacillus stearothermophilus NUB36 chromosome. J Bacteriol. 1990 Feb;172(2):793–801. doi: 10.1128/jb.172.2.793-801.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis R. C., Iwata K. K., Furlong C. E. Regulation of Glutamine Transport in Escherichia coli. J Bacteriol. 1975 Jun;122(3):1032–1037. doi: 10.1128/jb.122.3.1032-1037.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. J., Welker N. E. Protoplast transformation of Bacillus stearothermophilus NUB36 by plasmid DNA. J Gen Microbiol. 1989 May;135(5):1315–1324. doi: 10.1099/00221287-135-5-1315. [DOI] [PubMed] [Google Scholar]
- Wu L., Welker N. E. Temperature-induced protein synthesis in Bacillus stearothermophilus NUB36. J Bacteriol. 1991 Aug;173(15):4889–4892. doi: 10.1128/jb.173.15.4889-4892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis 168. J Bacteriol. 1973 Feb;113(2):540–548. doi: 10.1128/jb.113.2.540-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Nakai H., Imanaka T. Useful Host-Vector Systems in Bacillus stearothermophilus. Appl Environ Microbiol. 1988 Dec;54(12):3162–3164. doi: 10.1128/aem.54.12.3162-3164.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]