Abstract
Aims:
Sleep disordered breathing (SDB) is common in heart failure and ventilation is known to influence heart rate. Our aims were to assess the influence of SDB on heart rate variability (HRV) and to determine whether central sleep apnea (CSA) and obstructive sleep apnea (OSA) produced different patterns of HRV.
Methods and Results:
Overnight polysomnography was performed in 21 patients with heart failure and SDB. Two 10-minute segments each of SDB and stable breathing from each patient were visually identified and ECG signal exported for HRV analysis. SDB increased total power (TP) with very low frequency (VLF) power accounting for the greatest increase (1.89±0.54 vs 2.96±0.46 ms2, P <0.001); LF/HF ratio increased during SDB (1.2±1.0 vs 2.7±2.1, P <0.001). Compared to OSA, CSA was associated with lower absolute LF (2.10±0.47 vs 2.52±0.55 ms2, P = 0.049) and HF power (1.69±0.41 vs 2.34±0.58 ms2, P = 0.004), increased VLF% (78.9%±13.4% vs 60.9%±19.2%, P = 0.008), decreased HF% (6.9%±7.8% vs 16.0%±11.7%, P = 0.046) with a trend to higher LF/HF ratio.
Conclusions:
SDB increases HRV in the setting of increased sympathetic dominance. HRV in CSA and OSA have unique HRV patterns which are likely to reflect the different pathophysiological mechanisms involved.
Citation:
Szollosi I; Krum H; Kaye D; Naughton MT. Sleep apnea in heart failure increases heart rate variability and sympathetic dominance. SLEEP 2007;30(11):1509-1514.
Keywords: Congestive heart failure, heart rate variability, sleep apnea, cardio-respiratory coupling
INTRODUCTION
PATIENTS WITH CONGESTIVE HEART FAILURE (CHF) ARE KNOWN TO HAVE ABNORMAL HEART RATE VARIABILITY (HRV), NAMELY REDUCED TIME DOMAIN1 measures of HRV and reduced spectral power at all frequencies,2 with increased LF/HF ratio3 reflecting impaired parasympathetic control and increased sympathetic dominance. Such abnormalities in HRV have been consistently associated with poor prognosis.4,5
Measurements of HRV conducted during wakefulness 6,7 and sleep,8 indicate that during periodic breathing (PB) and Cheyne-Stokes Respiration (CSR), significant power and discrete peaks occur in the very low frequency band (VLF), corresponding to 0.01-0.04 Hz and the PB and CSR cycle length. The entrainment of heart rate and blood pressure by voluntary PB in normal subjects has also been shown to produce significant power in the VLF band, 9,10 and it is thought that these changes result from ventilation driven cardiopulmonary coupling rather than from a central vasomotor rhythm.7
PB and CSR are common during sleep and wakefulness in patients with CHF where instability in ventilatory control result from increased circulation time and heightened sensitivity of the chemoreceptors to hypercapnic and hypoxic stimuli.11,12 However, during sleep it is reported that an additional 10% to 25% of patients with CHF suffer from obstructive sleep apnea (OSA)13,14 which results from upper airway collapse, rather than instability in ventilatory control. OSA is a recognised risk factor for the development of hypertension and left untreated can accelerate the development and progression of cardiovascular disease.15 A decrease in heart rate during obstructive apneas and a relative tachycardia at the resumption of ventilation have also been described in OSA.16,17
Both central sleep apnea (CSA) with CSR, and to a lesser extent OSA, are associated with elevated awake plasma norepinephrine,18 overnight urinary norepinephrine,18–20 increased cardiac and total body tritiated norepinephrine spillover rate19 and awake muscle sympathetic nerve activity.21 Whether sympathetic activation increases progressively during sleep in the presence of breathing abnormalities or whether within-night changes in autonomic function occur with either CSA or OSA have not been investigated to date.
The aims of the present study were to describe the influence of SDB (OSA and CSA) on time and frequency domain measures of HRV in patients with CHF. Specifically, we sought to test the following hypotheses: first, that SDB will increase HRV in the setting of sympathetic dominance, as reflected by an increase in LF/HF ratio; second, that HRV abnormalities, in particular LF/HF ratio, will be more pronounced in the latter part of the night, reflecting greater sympathetic activation caused by SDB; and third, that CSA and OSA will produce different patterns of cardiovascular activation.
METHODS
Subjects
We evaluated 21 consecutive CHF patients referred for polysomnography for investigation of sleep disordered breathing. Inclusion criteria included following: aged 18-75 years; documented ischemic or idiopathic dilated cardiomyopathy ≥6 months duration with LVEF <45%; stable medical condition at time of assessment (no hospital admission or changes in medication for ≥2 weeks), sinus rhythm; and absence of significant co-existent medical conditions. Exclusion criteria were primary valvular, congenital, or restrictive cardiomyopathy; recent myocardial infarct (within 3 months); pregnancy; and inability to give informed consent.
For HRV analysis to be performed during sleep, patients had to be in sinus rhythm and segments chosen for HRV analyses free from excessive ectopic beats (<5%). In addition, all were required to have a minimum of 10-minute periods each of SDB and stable breathing during NREM sleep. The study was approved by the Alfred Hospital Ethics Committee, and all patients provided written informed consent.
Polysomnography
Overnight polysomnography was performed using Compumedics Profusion-2 (Compumedics E-series, Abbottsford, Australia) data acquisition and analysis system. Physiological signals monitored included EEG (C4-A1, C3-A2, O2-A1, O1-A2); EOG; submental EMG; ribcage and abdominal effort measured by piezo-electric sensors; body position measured by calibrated sensor; snoring sound measured with microphone; oro-nasal flow measured with thermistor and nasal pressure cannula (Pro-Tech Services, Washington, USA), SpO2(Oxypleth, Novametrix, Connecticut, USA) with averaging time set at 3 seconds; and PtcCO2 (TCM-3, Radiometer). ECG (lead II) was sampled at 512 Hz. Sleep stages and arousals were scored according to standard criteria.22,23 Respiratory events were scored as follows: apnea was defined as a cessation of airflow ≥10 seconds, classified as obstructive in the presence of continued movement in the respiratory bands and as central in the absence of movement in the respiratory bands. Hypopneas were defined as a >50% reduction in oronasal flow amplitude ≥10 seconds, accompanied by >2% desaturation and/or arousal. Hypopneas were classified as obstructive if there was evidence of upper airway resistance such as snoring, paradoxical motion in the respiratory bands,24 or inspiratory flow limitation on nasal pressure signal; and central if respiratory movements remained in phase and there was no evidence of inspiratory flow limitation. Patients were stratified into CSA and OSA groups depending on whether >50% of respiratory events were central or obstructive on origin.
Selection of Segments for HRV Analysis
For each subject, 2 conditions were examined—SDB and stable breathing. Where possible, 2 samples from each condition were obtained in order to assess both the influence of time of sampling and breathing condition on HRV. Thus a total of 4 segments were chosen for HRV analysis, 2 SDB segments and 2 stable segments from each patient.
Ten-minute segments were chosen by visual identification of the polysomnogram. The first and last 10-minute segments to meet the following criteria were used for HRV analysis: stable breathing was defined as no respiratory events or periodic leg movements occurring during the 10-minute period, absence of movement arousals, SpO2 range within 2%, and PtcCO2 range within 2 mm Hg. SDB was defined as continuous cycles of apnea or hypopnea during the 10-minute period, no arousals causing prolonged wakefulness (>1 epoch), and no changes in body position. All segments were chosen from NREM sleep, and the choice of segments was biased to be selected from Stage 2 sleep.
Once appropriate segments were identified, the ECG signal was exported for HRV analysis using the HRV module for Powerlab Chart (ADInstruments, Texas). R wave detection was performed using the maximum after threshold method, ectopics were excluded from the analysis, and standard time and frequency domain variables were calculated. Spectral analysis was performed using Fast Fourier Transform (FFT) with a spectrum size of 1024 points, applying a Parzen window with no overlap. Total Power (TP) was defined as 0-0.4Hz, Ultra Low Frequency (ULF) was 0-0.01Hz, Very Low Frequency (VLF) was 0.01-0.04 Hz, Low Frequency (LF) was 0.04-0.15 Hz, and High Frequency (HF) was 0.15-0.4 Hz for the purposes of the analysis. The normalized LF and HF power were calculated as LFnu=(LF/LF+HF)∗100 and HFnu=(HF/LF+HF)∗100. As LFnu and HFnu are inversely related to each other and determine the LF/HF ratio, these 3 indices are interrelated and can only provide a single interpretation regarding autonomic balance. As such, only the LF/HF ratio results will be presented. The relative distribution of power in each frequency band was calculated as a percentage of TP minus ULF component to give percentages of VLF, LF, and HF.
Statistical Analyses
HRV variables that were not normally distributed (TP, VLF, LF, and HF) were log transformed prior to statistical analysis and for data presentation. To assess differences in HRV during stable sleep between patients with predominantly OSA and CSA, we performed 2-tailed Student's t test on the first stable breathing period identified from each subject. To assess the influence of SDB and time of sampling on HRV, we performed linear regression analysis with time of sampling, breathing condition, and the interaction between condition and time of sampling included in the model. Where no interaction effects occurred, the interaction effect was removed from the regression model. To assess the differential effects of OSA and CSA, we performed multiple regression analyses with condition (stable breathing, OSA, and CSA) and sample (first and last) included in the model. All analyses were performed with robust standard errors adjusted for clustering by subject to address between subject and within subject sources of variability, using Stata9 (StataCorp LP, Texas, USA). All results are presented as mean + SD or n (%).
RESULTS
Patient characteristics are presented in Table 1 and indicate a middle-aged male predominance, with ischemic heart disease as the main cause of cardiomyopathy, and 86% of patients taking beta-blockers. The OSA and CSA groups were similar in most respects, however significantly greater BMI with a trend to greater LVEF was observed in the OSA group. Importantly, no significant differences were found during stable breathing in any HRV parameter measured between patients with predominantly OSA and CSA.
Table 1.
Subject Characteristics
| Subject Characteristics | OSA (n=8) | CSA (n=13) | P value |
|---|---|---|---|
| Age (y) | 55.2±5.2 | 52.4±15.6 | ns |
| Gender | |||
| M | 7 | 11 | |
| F | 1 | 2 | |
| CHF etiology | |||
| IHD | 4 | 9 | |
| DCM | 4 | 4 | |
| NYHA | |||
| I | 1 | 0 | |
| II | 2 | 3 | |
| III | 5 | 6 | |
| IV | 0 | 4 | |
| LVEF (%) | 33.1±9.4 | 26.7±7.8 | ns |
| BMI (kg/m2) | 31.7±4.0 | 26.2±4.2 | 0.008 |
| ESS | 8.1±3.2 | 8.6±3.5 | ns |
| AHI (/hr) | 20.6±10.0 | 22.3±13.1 | ns |
| Mean SpO2 | 96.9±0.8 | 96.4±1.0 | ns |
| Min SpO2 | 87.6±3.4 | 83.5±6.1 | ns |
| Medications | |||
| Diuretics | 4 (50%) | 13 (100%) | 0.003 |
| AT2R | 2 (29%) | 1 (8%) | ns |
| Digoxin | 2 (25%) | 4 (31%) | ns |
| Calcium Blocker | 3 (38%) | 2 (15%) | ns |
| Beta-blocker | 5 (62%) | 13 (100%) | 0.016 |
| ACE Inhibitor | 5 (62%) | 11 (85%) | ns |
| Amiodarone | 1 (12%) | 2 (15%) | ns |
(IHD=ischemic heart disease, DCM=dilated cardiomyopathy, NYHA=New York Heart Association classification, LVEF=left ventricular ejection fraction measured by gated blood pool scan, BMI=body mass index, ESS=Epworth Sleepiness Score, AHI=apnea-hypopnea index, AT2R=angiotensin II receptor antagonist)
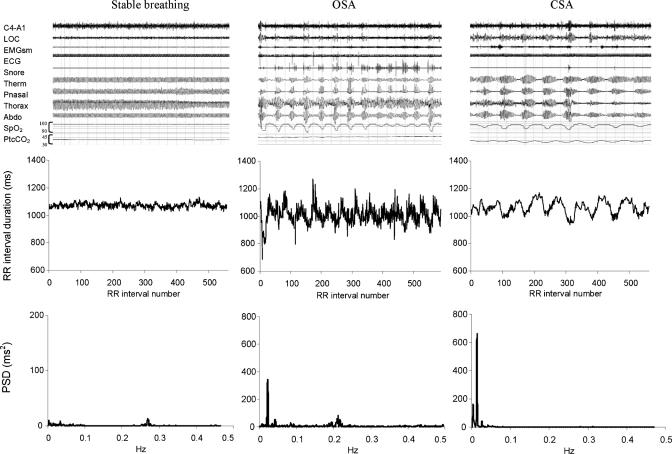
Fifteen patients had all 4 segments meeting our criteria for HRV analysis, 5 had three segments and 1 had two segments included in the analysis. Figure 1 (upper panels) illustrates typical 10-minute segments identified from polygraphs as stable breathing, OSA, and CSA. Heart rate tachographs and power spectral density plots for the corresponding segments are shown in the middle and lower panels, respectively. The typical OSA pattern of HRV was characterized by a VLF rhythm in the presence of continued HF oscillations; in contrast, the typical CSA pattern of HRV was a pronounced VLF rhythm and relatively blunted HF rhythm.
Figure 1.
Polysomnogram example (top panels), RR interval plot (middle panels), power spectral density plot (lower panels)
Comparison of Stable and Sleep Disordered Breathing
Group data for the comparison of SDB and stable breathing are presented in Table 2, with a total of 38 stable breathing segments and 39 SDB segments analysed. There was no influence of time of sampling on any time or frequency domain measures of HRV. Time domain measures of HR variance (i.e. standard deviation of RR interval (SDNN) and the range of RR interval (rangeNN)) were significantly influenced by breathing condition, showing a marked increase during SDB compared to stable breathing. The standard deviation of the difference in RR intervals (SDdNN), which is a time domain measure of rapid changes in HR, was also significantly increased during SDB compared with stable breathing.
Table 2.
Time and Frequency Domain Measures of HRV
| Stable | SDB | Time P | Breathing P | ||
|---|---|---|---|---|---|
| TIME DOMAIN | |||||
| mean HR (bpm) | First | 69.3±11.9 | 66.6±11.3 | ns | ns |
| Last | 65.1±11.4 | 66.1±11.7 | |||
| meanNN (ms) | First | 888±141 | 923±142 | ns | ns |
| Last | 948±164 | 932±150 | |||
| medianNN (ms) | First | 887±141 | 927±145 | ns | ns |
| Last | 948±164 | 934±154 | |||
| maxNN (ms) | First | 958±155 | 1044±168 | ns | <0.001 |
| Last | 1022±184 | 1049±158 | |||
| minNN (ms) | First | 824±135 | 780±112 | ns | <0.001 |
| Last | 875±144 | 789±123 | |||
| rangeNN (ms) | First | 134±66 | 264±119 | ns | <0.001 |
| Last | 147±86 | 288±118 | |||
| SDNN (ms) | First | 21.2±10.6 | 44.8±21.4 | ns | <0.001 |
| Last | 23.8±13.3 | 47.2±22.3 | |||
| SDdNN (ms) | First | 20.0±12.1 | 23.8±16.0 | ns | 0.019 |
| Last | 19.2±13.0 | 23.1±14.1 | |||
| NN50 (count) | First | 31.6±63.6 | 42.3±67.7 | ns | ns |
| Last | 25.8±49.3 | 36.3±63.9 | |||
| FREQUENCY DOMAIN | |||||
| TP ln (ms2) | First | 2.50±0.46 | 3.21±0.44 | ns | <0.001 |
| Last | 2.58±0.52 | 3.25±0.42 | |||
| VLF ln (ms2) | First | 1.78±0.48 | 2.92±0.46 | ns | <0.001 |
| Last | 1.99±0.59 | 3.01±0.47 | |||
| LF ln (ms2) | First | 1.75±0.57 | 2.36±0.54 | ns | <0.001 |
| Last | 1.87±0.65 | 2.25±0.57 | |||
| HF ln (ms2) | First | 1.90±0.58 | 2.06±0.60 | ns | ns |
| Last | 1.89±0.65 | 1.94±0.60 | |||
| LF/HF | First | 0.97±0.65 | 2.47±1.47 | ns | <0.001 |
| Last | 1.36±1.23 | 2.91±2.59 | |||
Obtained from the first and last stable breathing and SDB conditions. Influence of 2 factors, i.e., time and breathing were assessed using 2-way Repeated Measures ANOVA, hence 2 P-values are given.
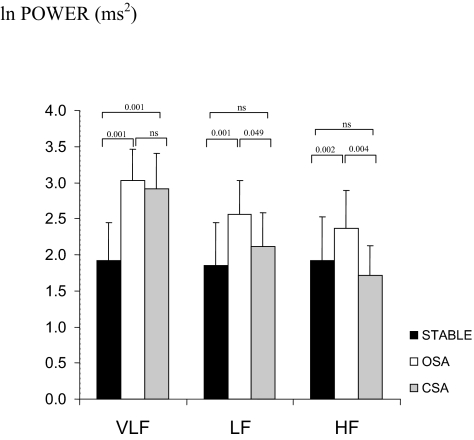
The frequency domain measure of TP was significantly increased during SDB compared with stable breathing. The increase in TP with SDB was largely driven by an increase in VLF power, as shown in Figure 2. There was an increase in normalized LF power (LFnu) and a decrease in normalized HF power (HFnu) leading to a net increase in the LF/HF ratio during SDB compared with the stable breathing.
Figure 2.
Absolute power distribution.
Comparison of OSA and CSA
The group data for the comparison of OSA and CSA are presented in Table 3. Importantly, there were no differences in any HRV measure obtained during stable breathing conditions between patients with predominantly OSA and CSA, thus pooled results are presented for stable breathing conditions. In the time domain, range NN, SDdNN, and NN50 were greater for OSA than CSA.
Table 3.
Time and Frequency Domain Measures of HRV with SDB Stratified into OSA and CSA Conditions
| Stable | OSA | CSA | P OSAvCSA | ||
|---|---|---|---|---|---|
| TIME DOMAIN | |||||
| mean HR (bpm) | 67.1±11.7 | 64.0±9.6 | 68.6±12.6 | ns | |
| meanNN (ms) | 920±155 | 956±135 | 900±152 | ns | |
| medianNN (ms) | 920±154 | 960±136 | 902±156 | ns | |
| maxNN (ms) | 992±172 | 1093±154 * | 1003±160 | ns | |
| minNN (ms) | 851±140 | 782±113 * | 786±122 * | ns | |
| rangeNN (ms) | 141±76 | 318±132 # | 217±76 ^ | 0.037 | |
| SDNN (ms) | 22.6±12.1 | 51.3±23.8 # | 40.9±18.4 # | ns | |
| SDdNN (ms) | 29.6±12.4 | 30.9±17.5 ^ | 16.4±7.1 | 0.020 | |
| NN50 (count) | 28.6±55.8 | 70.2±82.7 * | 10.1±12.2 | 0.032 | |
| FREQUENCY DOMAIN | |||||
| TP ln (ms2) | 2.54±0.48 | 3.33±0.40 # | 3.13±0.43 # | ns | |
| VLF ln (ms2) | 1.89±0.54 | 3.00±0.45 # | 2.92±0.47 # | ns | |
| LF ln (ms2) | 1.81±0.61 | 2.52±0.55 # | 2.10±0.47 | 0.049 | |
| HF ln (ms2) | 1.89±0.61 | 2.34±0.58 ^ | 1.69±0.41 | 0.004 | |
| LF/HF | 1.18±1.01 | 2.04±1.53 ^ | 3.29±2.36 # | ns | |
| VLF% | 34.5±11.9 | 60.9±19.2 # | 78.9±13.4 # | 0.008 | |
| LF% | 29.1±10.9 | 23.1±13.8 | 14.2±8.8 # | 0.048 | |
| HF% | 36.4±18.0 | 16.0±11.7 # | 6.9±7.8 # | 0.043 | |
OSA and CSA comparison to stable breathing shown by symbols P <0.05, ^P <0.01, #P <0.001. P OSAvCSA refers to OSA and CSA comparison.
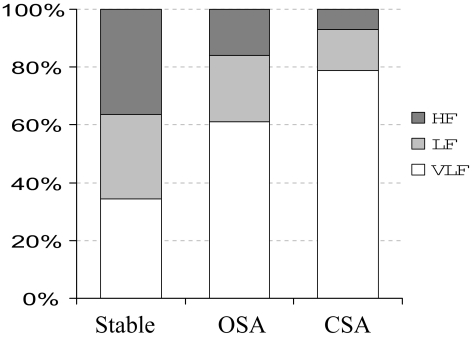
Figure 2 shows the absolute power contribution of VLF, LF, and HF frequency bands occurring with each condition. Absolute LF and HF power were significantly greater during OSA than CSA. Figure 3 shows the relative power contribution of all frequency bands occurring with each condition. VLF percentage was increased and HF percentage was reduced during CSA compared with OSA. The relative distribution of power in each frequency band for OSA was intermediate between stable breathing and CSA.
Figure 3.
Relative power distribution.
When LVEF or BMI were controlled for by inclusion in the regression model, OSA was associated with a significant increase in absolute HF power compared to CSA and stable breathing, while both conditions were associated with increased TP, VLF, and LF power. VLF percentage remained significantly greater during CSA than OSA.
DISCUSSION
This is the first study to examine the effects of spontaneous SDB compared to stable breathing on HRV in patients with stable heart failure during sleep. In addition, this is the first study to examine the differential effects of CSA and OSA on HRV in this patient group. There were three main findings of this study. First, the within-subject transition from stable breathing to SDB was associated with an increase in HRV, and in particular VLF power, as well as an increase in the LF/HF ratio; thus the increase in HRV occurred in the setting of increased sympathetic dominance. Second, we found no difference in HRV from samples taken from the beginning and end of the sleep period and importantly no evidence that cardiac autonomic regulation altered during the night independent of SDB. Finally, we found that OSA and CSA produced different patterns of HRV, with OSA showing increased absolute HF power and reduced VLF percentage compared with CSA. This relationship remained significant even when controlling for the possible confounding effect of differences in LVEF or BMI. Furthermore, the differences in HRV between OSA and CSA were most evident when the relative contribution of the VLF, LF, and HF rhythms to total power were assessed.
Our findings are consistent with previous observations that periodic breathing increases the VLF component of HRV. However earlier studies that examined the influence of periodic breathing on HRV were conducted during wakefulness6,7 and/or the comparisons to stable breathing conditions were achieved with interventions such as controlled breathing or CO2 administration.8 Nevertheless, these studies reported that VLF power is increased in patients with periodic breathing and CSA. Our findings extend these observations by showing that OSA also produces increased proportion of VLF power in patients with heart failure, although to a lesser extent than CSA.
By selecting SDB and stable breathing segments occurring spontaneously within the same subject, we were able to identify the precise influence of the abnormal breathing pattern on measures of HRV. The use of ten-minute segments in the present study was chosen to obtain adequate resolution of the VLF component, because the statistical properties of FFT based spectral analysis techniques improve when a period of at least 10 times the wavelength of interest is analysed.25 The selection of segments meeting the requirements of stationarity also reduces the possibility of spectral leakage contributing to the VLF component.
It has been suggested by previous investigators6 that prognostic information for mortality and cardiac events may be lost in the presence of breathing abnormalities, and the results of the present study support this notion. Patients with CHF are consistently found to have reductions in HRV, that is total power and power in all frequency bands.1,2 Reductions in VLF power are reported to be an independent predictor of poor outcome in this patient group.26 However, CSA has also been associated with poor prognosis;27,28 yet it causes an increase in VLF power. We propose that although CSA and OSA increase VLF power during periods of abnormal breathing, it is possible that HRV in the same patient may be blunted over longer term recordings, taking into account waking hours when ventilation is likely to be more stable. Indeed even during spontaneous stable breathing observed during sleep, our patients exhibited marked blunting in HRV. The interpretation of a single measure of HRV such as the SDNN or TP needs to be interpreted with caution as we have demonstrated that an overall increase in HRV may occur during a state of sympathetic dominance as reflected by the LF/HF ratio. Thus, interpretation of increased HRV cannot be generalized to indicate better autonomic control in this group of patients.
Other research groups have attempted to characterize HRV differences between OSA and CSA. For example, Spicuzza et al described the changes in HF and LF power during and after central and obstructive apneas in 17 non-CHF patients during sleep using 3-minute sample times.17 Obstructive apneas were characterized by 4-fold greater HF and 2-fold lower LF power than central apneas. Post-apnea HF fell in OSA and rose in CSA, whereas the LF rose in OSA and fell in CSA. The authors concluded that obstructive apneas resulted in an immediate post-apnea sympathetic surge, whereas the central apneas resulted in a post-apnea withdrawal of sympathetic activity. Importantly, there was an indication that the HF and LF patterns were different between the 2 apnea types, however VLF power was not assessed.
Despite increased HRV induced by repetitive cycles of apnea-hyperpea producing the characteristic surges in heart rate responsible for VLF rhythm, our results show that absolute HF power is increased during OSA compared with stable breathing and CSA. OSA results from repetitive upper airway collapse in the presence of continued respiratory effort; thus HF oscillations associated with respiratory sinus arrhythmia still occur, and to a greater degree than during stable breathing as large negative intrathoracic pressure swings can influence heart rate via direct and indirect mechanisms. CSA, conversely results from fluctuations in central brainstem efferent drive to the respiratory pump muscles during which respiratory sinus arrhythmia is diminished or absent during the apneic phase, thereby reducing oscillations in heart rate occurring in the HF band.
Although patients with predominantly OSA and CSA differed in clinical characteristics (notably BMI, medications, and possibly LVEF), the differences in HRV are unlikely to be attributed to clinical characteristics because during stable breathing condition, no differences were found between OSA and CSA patients in any HRV parameter measured. Thus the different patterns of HRV during OSA and CSA conditions are more likely to be the result of the type of SDB expressed during sleep than the clinical differences between groups. While LV dysfunction has a powerful influence on HRV, obesity has not been found to influence HRV29 or muscle sympathetic nerve activity independent of OSA.30 In addition, when we controlled for LVEF or BMI, the increase in HF power and decrease in VLF percentage during OSA compared with CSA remained significant.
In summary, this study of SDB in CHF has shown that SDB acutely causes an increase in HRV in the setting of increased sympathetic dominance and is the first study to show that OSA and CSA produce different patterns of HRV in patients with CHF. These results highlight that the interpretation of increased HRV cannot be generalized to indicate better autonomic control in this group of patients. Furthermore, the distinct pathophysiology of OSA and CSA produce differential effects on cardiovascular activation which become most evident when assessing the relative contribution of power in all three frequency bands.
Footnotes
Disclosure Statement
This is not an industry supported study. Dr. Szollosi has indicated ownership of intellectual property with provisional patent submitted for the use of heart rate variability to diagnose sleep disordered breathing. Dr. Naughton has served on the advisory board of ResMed; has received industry research support to assist Australian National Health and Medical Research sponsored clinical trials; and has submitted a provisional patent regarding the use of heart rate variability to diagnose sleep disordered breathing. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Casolo G, Balli E, Taddei T, Amuhasi J, Gori C. Decreased spontaneous heart rate variability in congestive heart failure. Am J Cardiol. 1989;64:1162–7. doi: 10.1016/0002-9149(89)90871-0. [DOI] [PubMed] [Google Scholar]
- 2.Saul JP, Arai Y, Berger RD, Lilly LS, Colucci WS, Cohen RJ. Assessment of autonomic regulation in chronic congestive heart failure by heart rate spectral analysis. Am J Cardiol. 1988;61:1292–9. doi: 10.1016/0002-9149(88)91172-1. [DOI] [PubMed] [Google Scholar]
- 3.Binkley PF, Nunziata E, Haas GJ, Nelson SD, Cody RJ. Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: demonstration in human subjects and verification in a paced canine model of ventricular failure. J Am Coll Cardiol. 1991;18:464–72. doi: 10.1016/0735-1097(91)90602-6. [DOI] [PubMed] [Google Scholar]
- 4.Lucreziotti S, Gavazzi A, Scelsi L, et al. Five-minute recording of heart rate variability in severe chronic heart failure: correlates with right ventricular function and prognostic implications. Am Heart J. 2000;139:1088–95. doi: 10.1067/mhj.2000.106168. [DOI] [PubMed] [Google Scholar]
- 5.Mortara A, Sleight P, Pinna GD, et al. Association between hemodynamic impairment and Cheyne-Stokes respiration and periodic breathing in chronic stable congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;84:900–4. doi: 10.1016/s0002-9149(99)00462-2. [DOI] [PubMed] [Google Scholar]
- 6.Mortara A, Sleight P, Pinna GD, et al. Abnormal awake respiratory patterns are common in chronic heart failure and may prevent evaluation of autonomic tone by measures of heart rate variability. Circulation. 1997;96:246–52. doi: 10.1161/01.cir.96.1.246. [DOI] [PubMed] [Google Scholar]
- 7.Pinna GD, Maestri R, Mortara A, La Rovere MT. Cardiorespiratory interactions during periodic breathing in awake chronic heart failure patients. Am J Physiol Heart Circ Physiol. 2000;278:H932–41. doi: 10.1152/ajpheart.2000.278.3.H932. [DOI] [PubMed] [Google Scholar]
- 8.Leung RS, Floras JS, Lorenzi-Filho G, Rankin F, Picton P, Bradley TD. Influence of Cheyne-Stokes respiration on cardiovascular oscillations in heart failure. Am J Respir Crit Care Med. 2003;167:1534–9. doi: 10.1164/rccm.200208-793OC. [DOI] [PubMed] [Google Scholar]
- 9.Francis DP, Davies LC, Willson K, Ponikowski P, Coats AJ, Piepoli M. Very-low-frequency oscillations in heart rate and blood pressure in periodic breathing: role of the cardiovascular limb of the hypoxic chemoreflex. Clin Sci (Lond) 2000;99:125–32. [PubMed] [Google Scholar]
- 10.Lorenzi-Filho G, Dajani HR, Leung RS, Floras JS, Bradley TD. Entrainment of blood pressure and heart rate oscillations by periodic breathing. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1147–54. doi: 10.1164/ajrccm.159.4.9806081. [DOI] [PubMed] [Google Scholar]
- 11.Andreas S. Nocturnal insights in chronic heart failure. Eur Heart J. 1999;20:1140–1. doi: 10.1053/euhj.1999.1643. [DOI] [PubMed] [Google Scholar]
- 12.Tremel F, Pepin JL, Veale D, et al. High prevalence and persistence of sleep apnoea in patients referred for acute left ventricular failure and medically treated over 2 months. Eur Heart J. 1999;20:1201–9. doi: 10.1053/euhj.1999.1546. [DOI] [PubMed] [Google Scholar]
- 13.Javaheri S, Parker T, Liming J, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 14.Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99:1574–9. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 15.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease. Cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 16.Guilleminault C, Connolly S, Winkle R, Melvin K, Tilkian A. Lancet. 1984. Cyclical variation of the heart rate in sleep apnoea syndrome. Mechanisms and usefulness of 24 h electrocardiography as a screening technique; pp. 126–31. [DOI] [PubMed] [Google Scholar]
- 17.Spicuzza L, Bernardi L, Calciati A, Di Maria GU. Autonomic modulation of heart rate during obstructive versus central apneas in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167:902–10. doi: 10.1164/rccm.200201-006OC. [DOI] [PubMed] [Google Scholar]
- 18.Naughton M, Benard D, Liu P, Rutherford R, Rankin F, Bradley T. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995;152:473–9. doi: 10.1164/ajrccm.152.2.7633695. [DOI] [PubMed] [Google Scholar]
- 19.Mansfield D, Kaye DM, Brunner La Rocca H, Solin P, Esler MD, Naughton MT. Raised sympathetic nerve activity in heart failure and central sleep apnea is due to heart failure severity. Circulation. 2003;107:1396–400. doi: 10.1161/01.cir.0000056520.17353.4f. [DOI] [PubMed] [Google Scholar]
- 20.Solin P, Kaye DM, Little PJ, Bergin P, Richardson M, Naughton MT. Impact of sleep apnea on sympathetic nervous system activity in heart failure. Chest. 2003;123:1119–26. doi: 10.1378/chest.123.4.1119. [DOI] [PubMed] [Google Scholar]
- 21.Spaak J, Egri ZJ, Kubo T, et al. Muscle sympathetic nerve activity during wakefulness in heart failure patients with and without sleep apnea. Hypertension. 2005;46:1327–32. doi: 10.1161/01.HYP.0000193497.45200.66. [DOI] [PubMed] [Google Scholar]
- 22.ASDA Task Force of the American Sleep Disorders Association. EEG arousals: scoring and examples. A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:174–84. [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Kales A. Washington DC: US Government Printing Office; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 24.Hirshkowitz M, Kryger M. Monitoring techniques for evaluating suspected sleep-disordered breathing. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: Elsevier Saunders; 2005. pp. 1378–93. [Google Scholar]
- 25.Task Force of the European Society of Cardiology the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 26.Hadase M, Azuma A, Zen K, et al. Very low frequency power of heart rate variability is a powerful predictor of clinical prognosis in patients with congestive heart failure. Circ J. 2004;68:343–7. doi: 10.1253/circj.68.343. [DOI] [PubMed] [Google Scholar]
- 27.Hanly P, Zuberi-Khokhar N. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153:272–6. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- 28.Lanfranchi A, Braghiroli A, Bosimini E, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–40. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 29.Antelmi I, De Paula RS, Shinzato AR, Peres CA, Mansur AJ, Grupi CJ. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol. 2004;93:381–5. doi: 10.1016/j.amjcard.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 30.Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiologica Scandinavica. 2003;177:385–90. doi: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]