Abstract
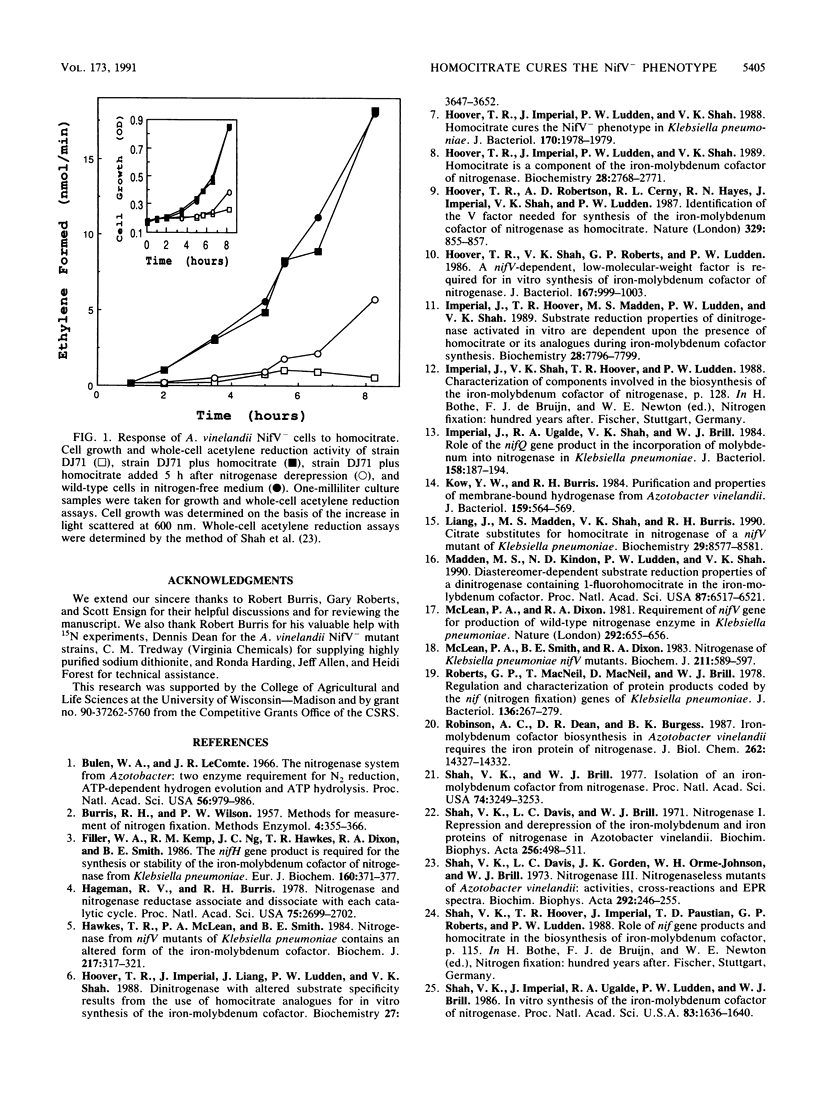
Azotobacter vinelandii DJ71, which contains a mutation in the nifV gene, was derepressed for nitrogenase in the presence of homocitrate. When dinitrogenase was isolated from this culture, it was found to be identical to the wild-type dinitrogenase. However, when the same NifV- strain was derepressed in the presence of erythrofluorohomocitrate, a homocitrate analog which produces a nitrogenase with wild-type properties in vitro, the isolated dinitrogenase was characteristic of the NifV- enzyme. These data show that homocitrate, but not fluorohomocitrate, is utilized by NifV- mutant cells. Fluorohomocitrate does not inhibit the uptake of homocitrate because the wild-type phenotype resulted when both compounds were added to the medium during nitrogenase derepression. Homocitrate lactone failed to cure the NifV- phenotype.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bulen W. A., LeComte J. R. The nitrogenase system from Azotobacter: two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc Natl Acad Sci U S A. 1966 Sep;56(3):979–986. doi: 10.1073/pnas.56.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler W. A., Kemp R. M., Ng J. C., Hawkes T. R., Dixon R. A., Smith B. E. The nifH gene product is required for the synthesis or stability of the iron-molybdenum cofactor of nitrogenase from Klebsiella pneumoniae. Eur J Biochem. 1986 Oct 15;160(2):371–377. doi: 10.1111/j.1432-1033.1986.tb09981.x. [DOI] [PubMed] [Google Scholar]
- Hageman R. V., Burris R. H. Nitrogenase and nitrogenase reductase associate and dissociate with each catalytic cycle. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2699–2702. doi: 10.1073/pnas.75.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes T. R., McLean P. A., Smith B. E. Nitrogenase from nifV mutants of Klebsiella pneumoniae contains an altered form of the iron-molybdenum cofactor. Biochem J. 1984 Jan 1;217(1):317–321. doi: 10.1042/bj2170317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover T. R., Imperial J., Liang J. H., Ludden P. W., Shah V. K. Dinitrogenase with altered substrate specificity results from the use of homocitrate analogues for in vitro synthesis of the iron-molybdenum cofactor. Biochemistry. 1988 May 17;27(10):3647–3652. doi: 10.1021/bi00410a019. [DOI] [PubMed] [Google Scholar]
- Hoover T. R., Imperial J., Ludden P. W., Shah V. K. Homocitrate cures the NifV- phenotype in Klebsiella pneumoniae. J Bacteriol. 1988 Apr;170(4):1978–1979. doi: 10.1128/jb.170.4.1978-1979.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover T. R., Imperial J., Ludden P. W., Shah V. K. Homocitrate is a component of the iron-molybdenum cofactor of nitrogenase. Biochemistry. 1989 Apr 4;28(7):2768–2771. doi: 10.1021/bi00433a004. [DOI] [PubMed] [Google Scholar]
- Hoover T. R., Robertson A. D., Cerny R. L., Hayes R. N., Imperial J., Shah V. K., Ludden P. W. Identification of the V factor needed for synthesis of the iron-molybdenum cofactor of nitrogenase as homocitrate. 1987 Oct 29-Nov 4Nature. 329(6142):855–857. doi: 10.1038/329855a0. [DOI] [PubMed] [Google Scholar]
- Hoover T. R., Shah V. K., Roberts G. P., Ludden P. W. nifV-dependent, low-molecular-weight factor required for in vitro synthesis of iron-molybdenum cofactor of nitrogenase. J Bacteriol. 1986 Sep;167(3):999–1003. doi: 10.1128/jb.167.3.999-1003.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial J., Hoover T. R., Madden M. S., Ludden P. W., Shah V. K. Substrate reduction properties of dinitrogenase activated in vitro are dependent upon the presence of homocitrate or its analogues during iron-molybdenum cofactor synthesis. Biochemistry. 1989 Sep 19;28(19):7796–7799. doi: 10.1021/bi00445a040. [DOI] [PubMed] [Google Scholar]
- Imperial J., Ugalde R. A., Shah V. K., Brill W. J. Role of the nifQ gene product in the incorporation of molybdenum into nitrogenase in Klebsiella pneumoniae. J Bacteriol. 1984 Apr;158(1):187–194. doi: 10.1128/jb.158.1.187-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow Y. W., Burris R. H. Purification and properties of membrane-bound hydrogenase from Azotobacter vinelandii. J Bacteriol. 1984 Aug;159(2):564–569. doi: 10.1128/jb.159.2.564-569.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Madden M., Shah V. K., Burris R. H. Citrate substitutes for homocitrate in nitrogenase of a nifV mutant of Klebsiella pneumoniae. Biochemistry. 1990 Sep 18;29(37):8577–8581. doi: 10.1021/bi00489a011. [DOI] [PubMed] [Google Scholar]
- Madden M. S., Kindon N. D., Ludden P. W., Shah V. K. Diastereomer-dependent substrate reduction properties of a dinitrogenase containing 1-fluorohomocitrate in the iron-molybdenum cofactor. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6517–6521. doi: 10.1073/pnas.87.17.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean P. A., Dixon R. A. Requirement of nifV gene for production of wild-type nitrogenase enzyme in Klebsiella pneumoniae. Nature. 1981 Aug 13;292(5824):655–656. doi: 10.1038/292655a0. [DOI] [PubMed] [Google Scholar]
- McLean P. A., Smith B. E., Dixon R. A. Nitrogenase of Klebsiella pneumoniae nifV mutants. Biochem J. 1983 Jun 1;211(3):589–597. doi: 10.1042/bj2110589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P., MacNeil T., MacNeil D., Brill W. J. Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):267–279. doi: 10.1128/jb.136.1.267-279.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. C., Dean D. R., Burgess B. K. Iron-molybdenum cofactor biosynthesis in Azotobacter vinelandii requires the iron protein of nitrogenase. J Biol Chem. 1987 Oct 15;262(29):14327–14332. [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Davis I. C., Gordon J. K., Orme-Johnson W. H., Brill W. J. Nitrogenase. 3. Nitrogenaseless mutants of Azotobacter vinelandii: activities, cross-reactions and EPR spectra. Biochim Biophys Acta. 1973 Jan 18;292(1):246–255. doi: 10.1016/0005-2728(73)90269-7. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis L. C., Brill W. J. Nitrogenase. I. Repression and derepression of the iron-molybdenum and iron proteins of nitrogenase in Azotobacter vinelandii. Biochim Biophys Acta. 1972 Feb 28;256(2):498–511. doi: 10.1016/0005-2728(72)90078-3. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Imperial J., Ugalde R. A., Ludden P. W., Brill W. J. In vitro synthesis of the iron-molybdenum cofactor of nitrogenase. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1636–1640. doi: 10.1073/pnas.83.6.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]



