Abstract
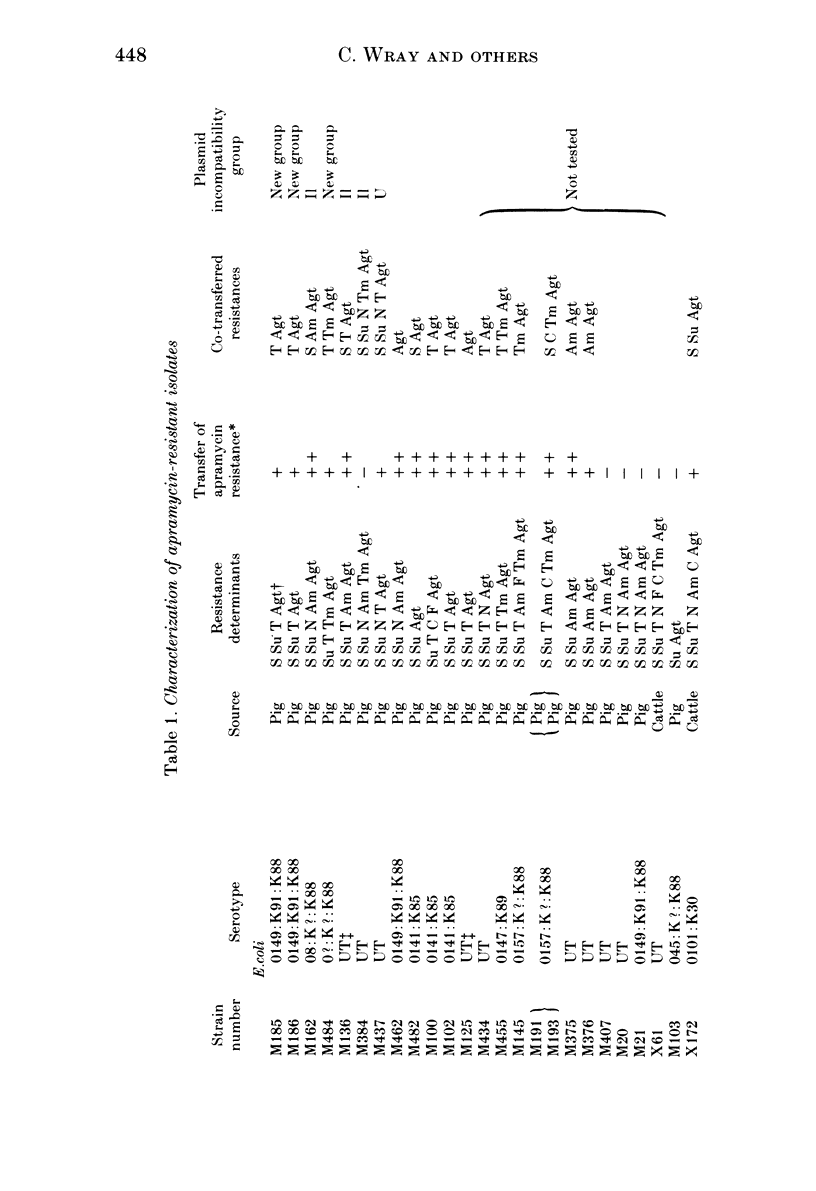
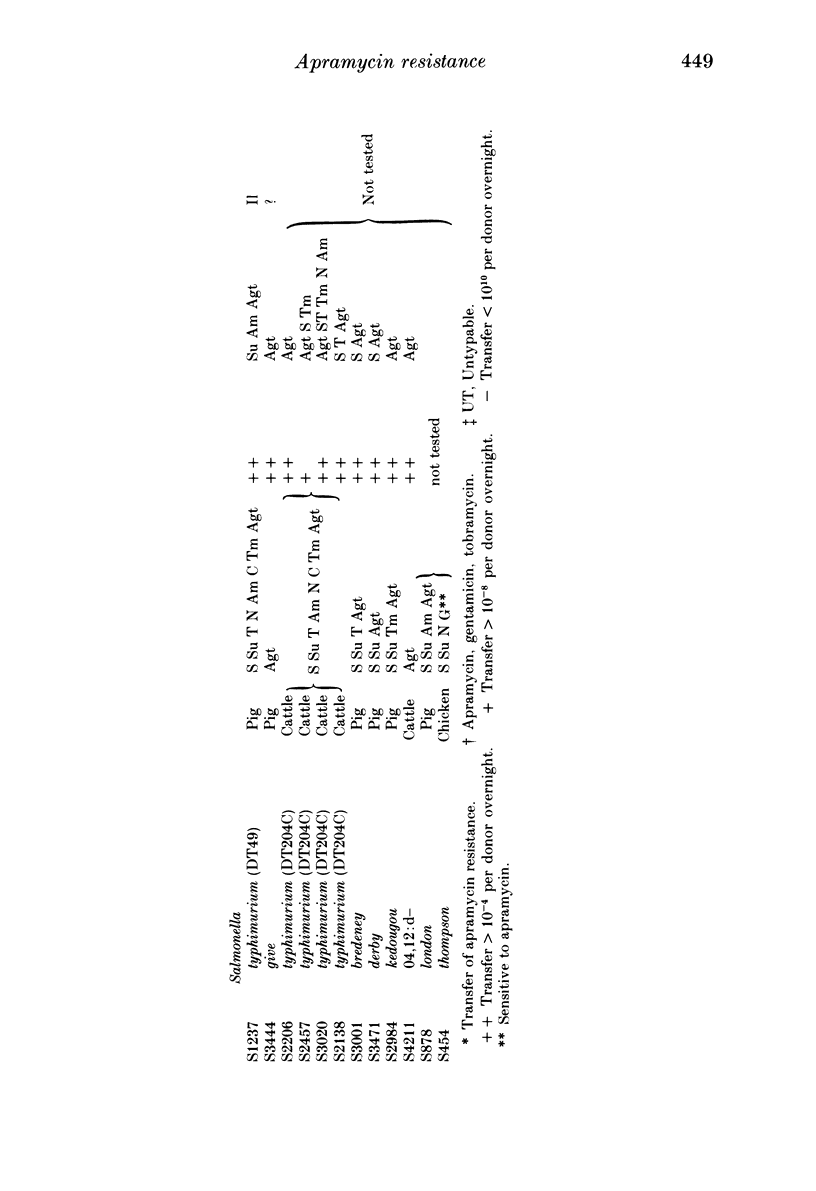
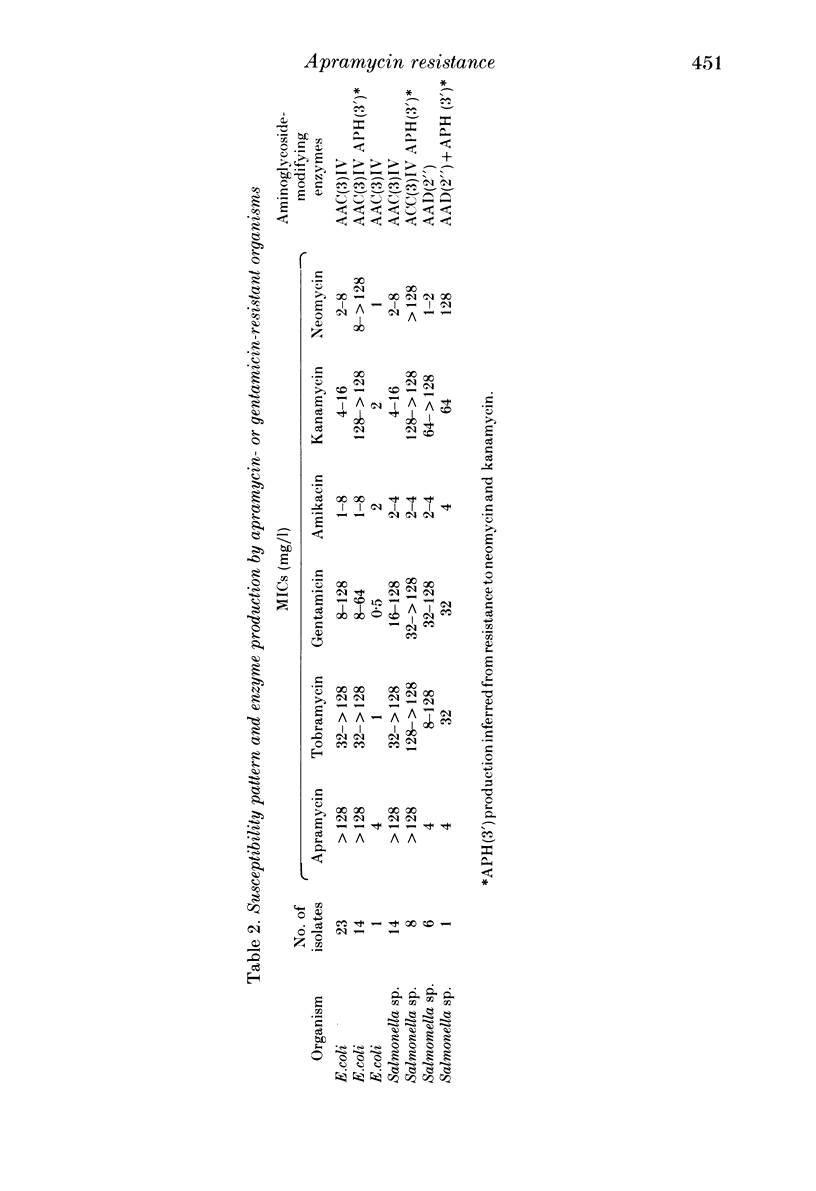
Since the aminoglycoside antibiotic apramycin was licensed for veterinary use in 1980, all isolates of Escherichia coli and salmonellas received at the Central Veterinary Laboratory have been monitored for resistance to apramycin and the related antibiotic gentamicin. During the period 1982-4, the incidence of resistance in E. coli to apramycin increased from 0.6% in 1982 to 2.6% in 1984. In salmonellas the incidence of resistance to apramycin increased from 0.1% in 1982 to 1.4% in 1984. Resistance to both apramycin and gentamicin was detected in six different salmonella serotypes, although an isolate of Salmonella thompson from poultry was resistant to gentamicin but not apramycin. Most of the cultures were isolated from pigs, although the incidence of apramycin resistance in S. typhimurium (DT 204C) from calves has shown a recent dramatic increase. All the isolates with one exception produced the enzyme aminoglycoside 3-N-acetyltransferase IV (ACC(3)IV). The resistance was transferable by conjugation in most of the strains examined, and the plasmids specifying the resistance have been found to belong to a number of different incompatibility groups. Plasmids from three E. coli strains were compatible with all the reference plasmids and belonged to a previously undescribed group which was investigated further. It is suggested that bacteria from humans should be examined for resistance to apramycin and gentamicin to determine the possibility of the antibiotic-resistance bacteria, and their genes, spreading from animals to humans.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. E. Morphological and serological relationships of conjugative pili. Plasmid. 1980 Sep;4(2):155–169. doi: 10.1016/0147-619x(80)90005-0. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Transfer systems of K88 and K99 plasmids. Plasmid. 1985 Mar;13(2):118–128. doi: 10.1016/0147-619x(85)90064-2. [DOI] [PubMed] [Google Scholar]
- Chaslus-Dancla E., Lafont J. P. Resistance to gentamicin and apramycin in Escherichia coli from calves in France. Vet Rec. 1985 Jul 27;117(4):90–91. doi: 10.1136/vr.117.4.90. [DOI] [PubMed] [Google Scholar]
- Datta N., Dacey S., Hughes V., Knight S., Richards H., Williams G., Casewell M., Shannon K. P. Distribution of genes for trimethoprim and gentamicin resistance in bacteria and their plasmids in a general hospital. J Gen Microbiol. 1980 Jun;118(2):495–508. doi: 10.1099/00221287-118-2-495. [DOI] [PubMed] [Google Scholar]
- Davies J., O'Connor S. Enzymatic modification of aminoglycoside antibiotics: 3-N-acetyltransferase with broad specificity that determines resistance to the novel aminoglycoside apramycin. Antimicrob Agents Chemother. 1978 Jul;14(1):69–72. doi: 10.1128/aac.14.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Baron L. S. EPISOMIC ELEMENT IN A STRAIN OF SALMONELLA TYPHOSA. J Bacteriol. 1962 Sep;84(3):581–589. doi: 10.1128/jb.84.3.581-589.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Shannon K. P. Resistance to apramycin in Escherichia coli isolated from animals: detection of a novel aminoglycoside-modifying enzyme. J Gen Microbiol. 1984 Mar;130(3):473–482. doi: 10.1099/00221287-130-3-473. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., FitzGerald G. B., Macone A. B. Spread of antibiotic-resistant plasmids from chicken to chicken and from chicken to man. Nature. 1976 Mar 4;260(5546):40–42. doi: 10.1038/260040a0. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. F., Hopkins J. D., Gilleece E. S., Medeiros A. A., Kent R. L., Blackburn B. O., Holmes M. B., Reardon J. P., Vergeront J. M., Schell W. L. Molecular epidemiology of antibiotic resistance in salmonella from animals and human beings in the United States. N Engl J Med. 1982 Jul 1;307(1):1–6. doi: 10.1056/NEJM198207013070101. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Benveniste R., Tipper D., Davies J. Aminoglycoside antibiotics: inactivation by phosphorylation in Escherichia coli carrying R factors. J Bacteriol. 1969 Nov;100(2):1144–1146. doi: 10.1128/jb.100.2.1144-1146.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price K. E., Kresel P. A., Farchione L. A., Siskin S. B., Karpow S. A. Epidemiological studies of aminoglycoside resistance in the U.S.A. J Antimicrob Chemother. 1981 Jul;8 (Suppl A):89–105. doi: 10.1093/jac/8.suppl_a.89. [DOI] [PubMed] [Google Scholar]
- Ryden R., Moore B. J. The in vitro activity of apramycin, a new aminocyclitol antibiotic. J Antimicrob Chemother. 1977 Nov;3(6):609=13–609=13. doi: 10.1093/jac/3.6.609. [DOI] [PubMed] [Google Scholar]
- Scotland S. M., Gross R. J., Cheasty T., Rowe B. The occurrence of plasmids carrying genes for both enterotoxin production and drug resistance in Escherichia coli of human origin. J Hyg (Lond) 1979 Dec;83(3):531–538. doi: 10.1017/s0022172400026383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley P. L., Gyles C. L., Falkow S. Characterization of plasmids that encode for the K88 colonization antigen. Infect Immun. 1978 May;20(2):559–566. doi: 10.1128/iai.20.2.559-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojka W. J., Wray C., McLaren I. A survey of drug resistance in salmonellae isolated from animals in England and Wales from 1979 to 1981. Br Vet J. 1984 Nov-Dec;140(6):576–591. doi: 10.1016/0007-1935(84)90009-5. [DOI] [PubMed] [Google Scholar]
- Threlfall E. J., Rowe B., Ferguson J. L., Ward L. R. Increasing incidence of resistance to gentamicin and related aminoglycosides in Salmonella typhimurium phage type 204c in England, Wales and Scotland. Vet Rec. 1985 Oct 5;117(14):355–357. doi: 10.1136/vr.117.14.355. [DOI] [PubMed] [Google Scholar]
- Witchitz J. L. Epidemiological aspects of aminoglycoside resistance in France. J Antimicrob Chemother. 1981 Jul;8 (Suppl A):71–82. doi: 10.1093/jac/8.suppl_a.71. [DOI] [PubMed] [Google Scholar]
- Wray C. Is salmonellosis still a serious problem in veterinary practice? Vet Rec. 1985 May 4;116(18):485–489. doi: 10.1136/vr.116.18.485. [DOI] [PubMed] [Google Scholar]



