Abstract
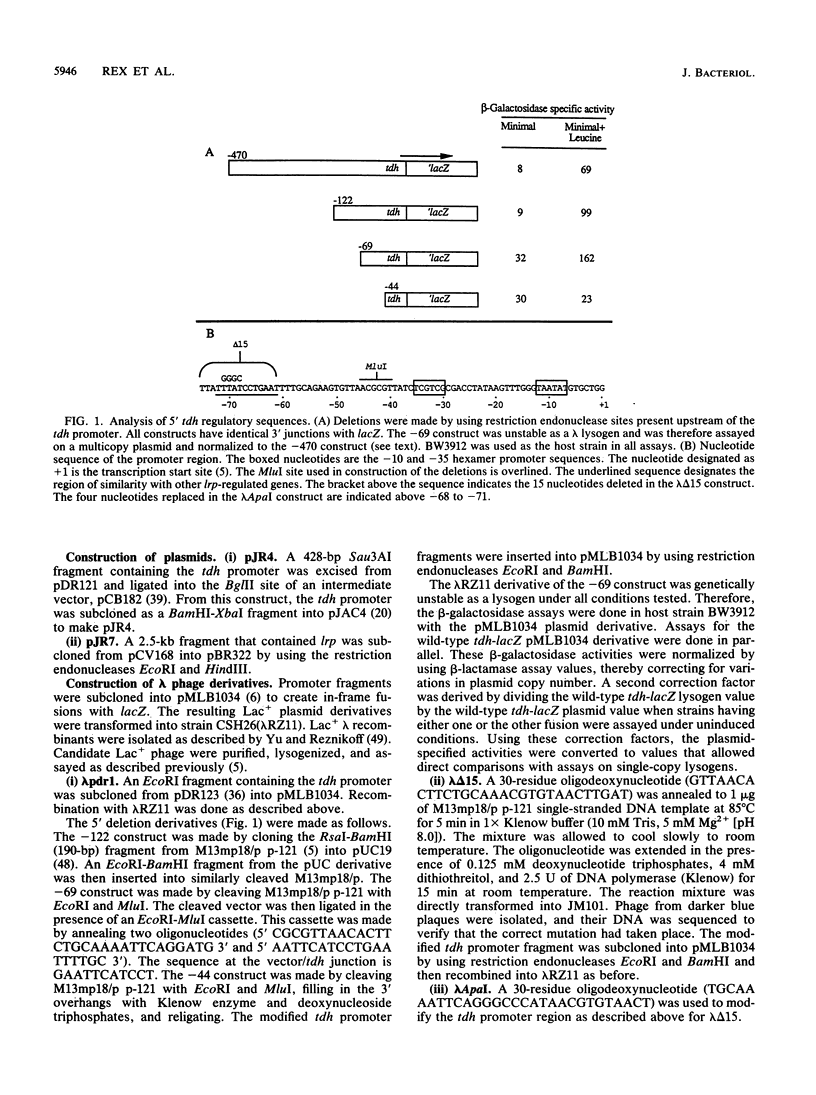
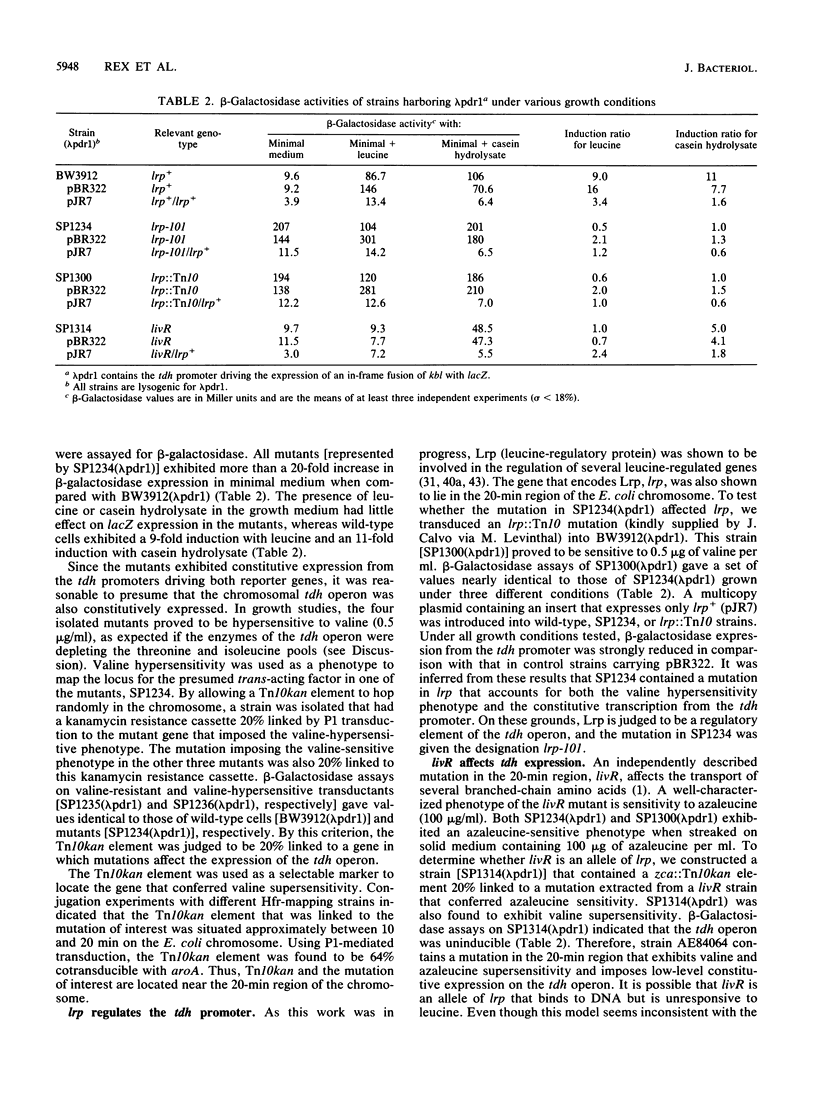
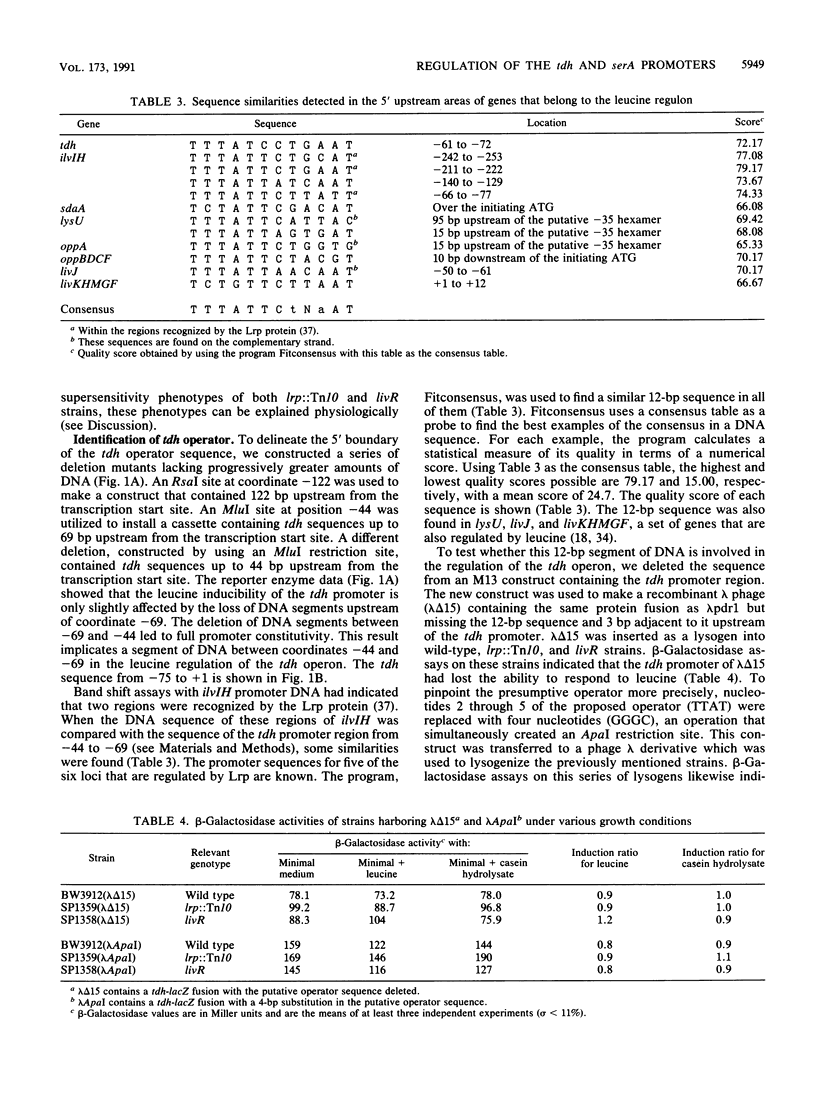
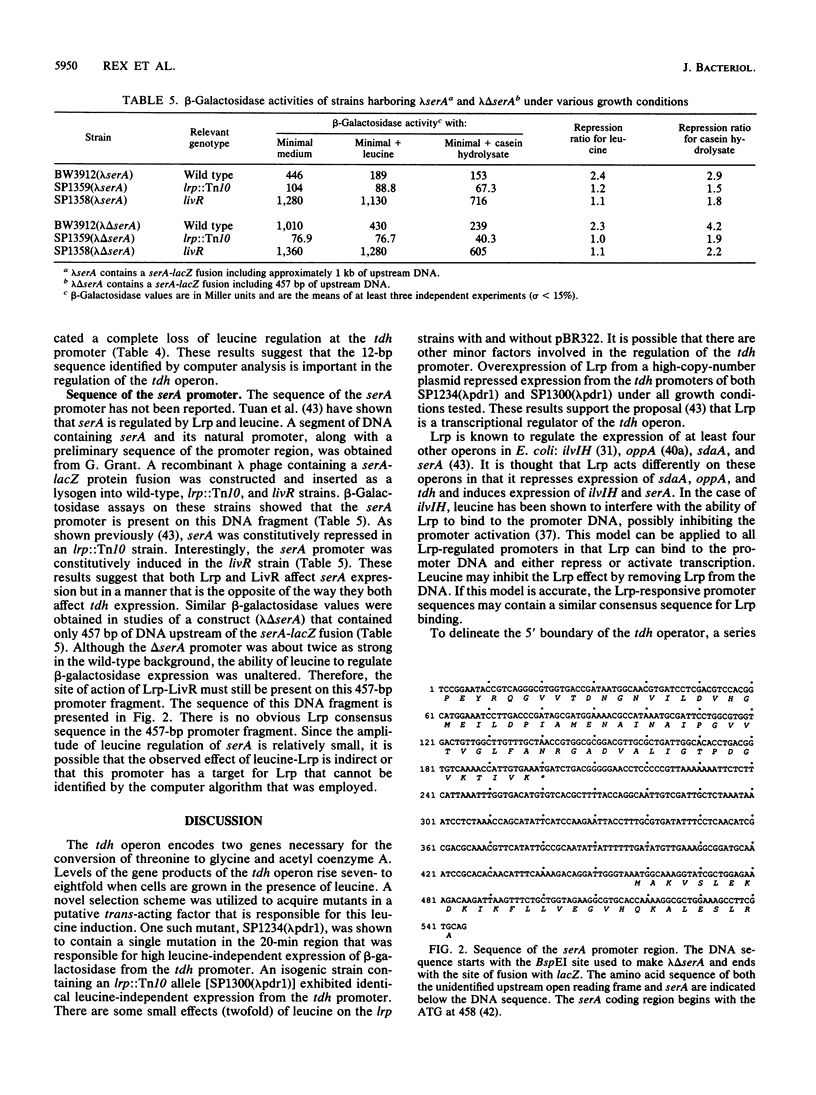
The tdh promoter of Escherichia coli is induced seven- to eightfold when cells are grown in the presence of exogenous leucine. A scheme was devised to select mutants that exhibited high constitutive expression of the tdh promoter. The mutations in these strains were shown to lie within a previously identified gene (lrp) that encodes Lrp (leucine-responsive regulatory protein). By deletion analysis, the site of action of Lrp was localized to a 25-bp region between coordinates -69 and -44 of the tdh promoter. Disruption of a 12-bp presumptive target sequence found in this region of tdh resulted in constitutively derepressed expression from the tdh promoter. Similar DNA segments (consensus, TTTATTCtNaAT) were also identified in a number of other promoters, including each of the Lrp-regulated promoters whose nucleotide sequence is known. The sequence of the promoter region of serA, an Lrp-regulated gene, was determined. No Lrp consensus target sequence was present upstream of serA, suggesting that Lrp acts indirectly on the serA promoter. A previously described mutation in a leucine-responsive trans-acting factor, LivR (J. J. Anderson, S. C. Quay, and D. L. Oxender, J. Bacteriol. 126:80-90, 1976), resulted in constitutively repressed expression from the tdh promoter and constitutively induced expression from the serA promoter. The possibility that LivR and Lrp are allelic is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Quay S. C., Oxender D. L. Mapping of two loci affecting the regulation of branched-chain amino acid transport in Escherichia coli K-12. J Bacteriol. 1976 Apr;126(1):80–90. doi: 10.1128/jb.126.1.80-90.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. J., Wilson J. M., Oxender D. L. Defective transport and other phenotypes of a periplasmic "leaky" mutant of Escherichia coli K-12. J Bacteriol. 1979 Nov;140(2):351–358. doi: 10.1128/jb.140.2.351-358.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J. C., Short S. A. opp-lac Operon fusions and transcriptional regulation of the Escherichia coli trp-linked oligopeptide permease. J Bacteriol. 1986 Feb;165(2):434–442. doi: 10.1128/jb.165.2.434-442.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci T. K., Wagner L. M., Oxender D. L. Cloning, expression, and nucleotide sequence of livR, the repressor for high-affinity branched-chain amino acid transport in Escherichia coli. Proteins. 1986 Oct;1(2):125–133. doi: 10.1002/prot.340010204. [DOI] [PubMed] [Google Scholar]
- Aronson B. D., Levinthal M., Somerville R. L. Activation of a cryptic pathway for threonine metabolism via specific IS3-mediated alteration of promoter structure in Escherichia coli. J Bacteriol. 1989 Oct;171(10):5503–5511. doi: 10.1128/jb.171.10.5503-5511.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M. L., Jackson D. E. Selection of lac gene fusions in vivo: ompR-lacZ fusions that define a functional domain of the ompR gene product. J Bacteriol. 1984 Aug;159(2):750–756. doi: 10.1128/jb.159.2.750-756.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Broman R. L., Goldenbaum P. E., Dobrogosz W. J. The effect of amino acids on the ability of cyclic AMP to reverse catabolite repression in Escherichia coli. Biochem Biophys Res Commun. 1970 May 11;39(3):401–406. doi: 10.1016/0006-291x(70)90591-7. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M., Levinthal M. The acetohydroxy acid synthase III isoenzyme of Escherichia coli K-12: regulation of synthesis by leucine. Biochem Biophys Res Commun. 1977 Nov 7;79(1):82–87. doi: 10.1016/0006-291x(77)90063-8. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J., Newman E. B. Derivation of glycine from threonine in Escherichia coli K-12 mutants. J Bacteriol. 1975 Jun;122(3):810–817. doi: 10.1128/jb.122.3.810-817.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. Integration-negative mutants of bacteriophage lambda. J Mol Biol. 1968 Feb 14;31(3):487–505. doi: 10.1016/0022-2836(68)90423-3. [DOI] [PubMed] [Google Scholar]
- Greene R. C., Radovich C. Role of methionine in the regulation of serine hydroxymethyltransferase in Eschericia coli. J Bacteriol. 1975 Oct;124(1):269–278. doi: 10.1128/jb.124.1.269-278.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H., Shimamoto T., Tsuda M., Tsuchiya T. Characterization of a novel L-serine transport system in Escherichia coli. J Bacteriol. 1988 May;170(5):2236–2239. doi: 10.1128/jb.170.5.2236-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L. I., Christensen H. N., Handlogten M. E., Oxender D. L., Quay S. C. Transport of L-4-azaleucine in Escherichia coli. J Bacteriol. 1975 Jun;122(3):957–965. doi: 10.1128/jb.122.3.957-965.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Yeh F. M., Sawyer L. E. Metabolites influence control of lysine transfer ribonucleic acid synthetase formation in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1364–1367. doi: 10.1073/pnas.72.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaurin B. A promoter probe vector (pJAC4) that utilizes the ampC beta-lactamase gene of Escherichia coli. Nucleic Acids Res. 1987 Oct 26;15(20):8567–8567. doi: 10.1093/nar/15.20.8567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasian P. A., Davidson B. E., Pittard J. Molecular analysis of the promoter operator region of the Escherichia coli K-12 tyrP gene. J Bacteriol. 1986 Aug;167(2):556–561. doi: 10.1128/jb.167.2.556-561.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. A., Frère J. M., Duez C., Ghuysen J. M. Interactions between non-classical beta-lactam compounds and the beta-lactamases of Actinomadura R39 and Streptomyces albus G. Biochem J. 1981 Oct 1;199(1):137–143. doi: 10.1042/bj1990137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger M., Wahl R., Rice P. Compilation of DNA sequences of Escherichia coli (update 1990). Nucleic Acids Res. 1990 Apr 25;18 (Suppl):2549–2587. doi: 10.1093/nar/18.suppl.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölling R., Lother H. AsnC: an autogenously regulated activator of asparagine synthetase A transcription in Escherichia coli. J Bacteriol. 1985 Oct;164(1):310–315. doi: 10.1128/jb.164.1.310-315.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R. G., Neidhardt F. C. Abnormal induction of heat shock proteins in an Escherichia coli mutant deficient in adenosylmethionine synthetase activity. J Bacteriol. 1988 Apr;170(4):1582–1588. doi: 10.1128/jb.170.4.1582-1588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKitrick J. C., Pizer L. I. Regulation of phosphoglycerate dehydrogenase levels and effect on serine synthesis in Escherichia coli K-12. J Bacteriol. 1980 Jan;141(1):235–245. doi: 10.1128/jb.141.1.235-245.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. B., Kapoor V., Potter R. Role of L-threonine dehydrogenase in the catabolism of threonine and synthesis of glycine by Escherichia coli. J Bacteriol. 1976 Jun;126(3):1245–1249. doi: 10.1128/jb.126.3.1245-1249.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platko J. V., Willins D. A., Calvo J. M. The ilvIH operon of Escherichia coli is positively regulated. J Bacteriol. 1990 Aug;172(8):4563–4570. doi: 10.1128/jb.172.8.4563-4570.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter R., Kapoor V., Newman E. B. Role of threonine dehydrogenase in Escherichia coli threonine degradation. J Bacteriol. 1977 Nov;132(2):385–391. doi: 10.1128/jb.132.2.385-391.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Dick T. E., Oxender D. L. Role of transport systems in amino acid metabolism: leucine toxicity and the branched-chain amino acid transport systems. J Bacteriol. 1977 Mar;129(3):1257–1265. doi: 10.1128/jb.129.3.1257-1265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Kline E. L., Oxender D. L. Role of leucyl-tRNA synthetase in regulation of branched-chain amino-acid transport. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3921–3924. doi: 10.1073/pnas.72.10.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnikar P. D., Somerville R. L. Genetic characterization of a highly efficient alternate pathway of serine biosynthesis in Escherichia coli. J Bacteriol. 1987 Jun;169(6):2611–2617. doi: 10.1128/jb.169.6.2611-2617.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnikar P. D., Somerville R. L. Structural and functional analysis of a cloned segment of Escherichia coli DNA that specifies proteins of a C4 pathway of serine biosynthesis. J Bacteriol. 1987 Oct;169(10):4716–4721. doi: 10.1128/jb.169.10.4716-4721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca E., Aker D. A., Calvo J. M. A protein that binds to the regulatory region of the Escherichia coli ilvIH operon. J Bacteriol. 1989 Mar;171(3):1658–1664. doi: 10.1128/jb.171.3.1658-1664.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K., Beck C. F. Promoter-probe vectors for the analysis of divergently arranged promoters. Gene. 1986;42(1):37–48. doi: 10.1016/0378-1119(86)90148-4. [DOI] [PubMed] [Google Scholar]
- Searles L. L., Wessler S. R., Calvo J. M. Transcription attenuation is the major mechanism by which the leu operon of Salmonella typhimurium is controlled. J Mol Biol. 1983 Jan 25;163(3):377–394. doi: 10.1016/0022-2836(83)90064-5. [DOI] [PubMed] [Google Scholar]
- Su H. S., Lang B. F., Newman E. B. L-serine degradation in Escherichia coli K-12: cloning and sequencing of the sdaA gene. J Bacteriol. 1989 Sep;171(9):5095–5102. doi: 10.1128/jb.171.9.5095-5102.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey K. L., Grant G. A. The nucleotide sequence of the serA gene of Escherichia coli and the amino acid sequence of the encoded protein, D-3-phosphoglycerate dehydrogenase. J Biol Chem. 1986 Sep 15;261(26):12179–12183. [PubMed] [Google Scholar]
- Tuan L. R., D'Ari R., Newman E. B. The leucine regulon of Escherichia coli K-12: a mutation in rblA alters expression of L-leucine-dependent metabolic operons. J Bacteriol. 1990 Aug;172(8):4529–4535. doi: 10.1128/jb.172.8.4529-4535.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wanner B. L. Novel regulatory mutants of the phosphate regulon in Escherichia coli K-12. J Mol Biol. 1986 Sep 5;191(1):39–58. doi: 10.1016/0022-2836(86)90421-3. [DOI] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Willins D. A., Ryan C. W., Platko J. V., Calvo J. M. Characterization of Lrp, and Escherichia coli regulatory protein that mediates a global response to leucine. J Biol Chem. 1991 Jun 15;266(17):10768–10774. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yu X. M., Reznikoff W. S. Deletion analysis of the CAP-cAMP binding site of the Escherichia coli lactose promoter. Nucleic Acids Res. 1984 Jul 11;12(13):5449–5464. doi: 10.1093/nar/12.13.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wind N., de Jong M., Meijer M., Stuitje A. R. Site-directed mutagenesis of the Escherichia coli chromosome near oriC: identification and characterization of asnC, a regulatory element in E. coli asparagine metabolism. Nucleic Acids Res. 1985 Dec 20;13(24):8797–8811. doi: 10.1093/nar/13.24.8797. [DOI] [PMC free article] [PubMed] [Google Scholar]


