Abstract
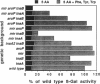
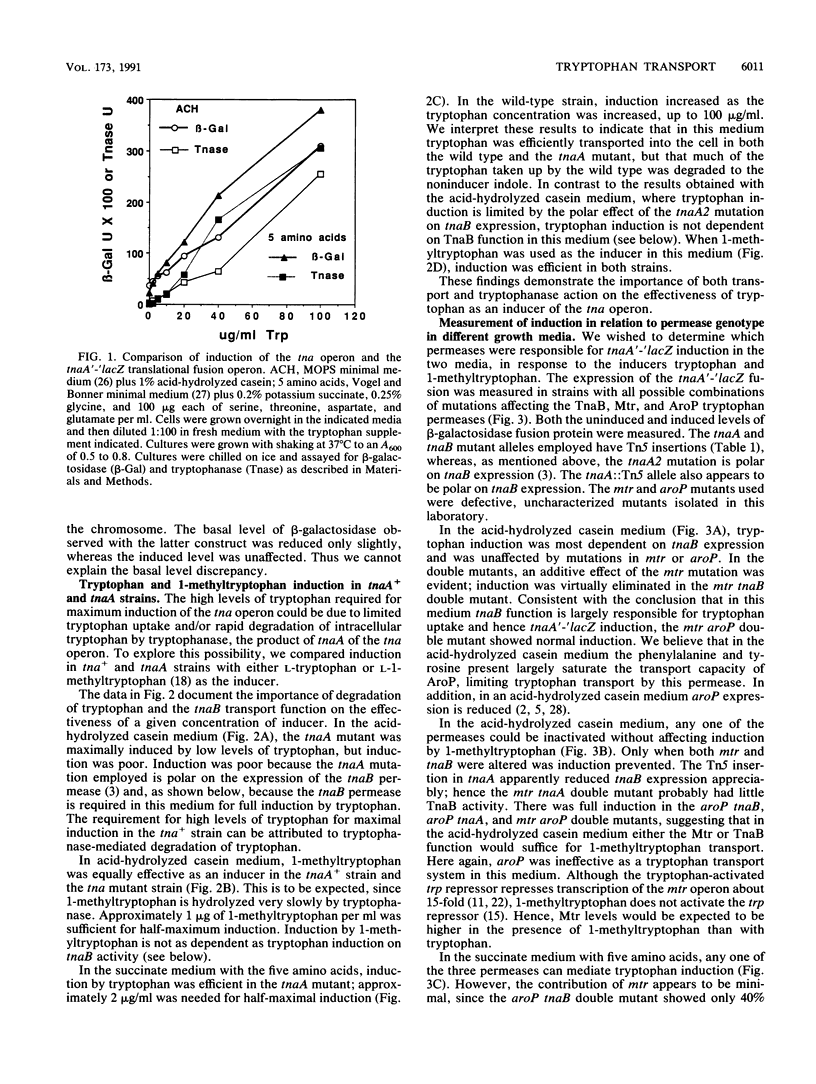
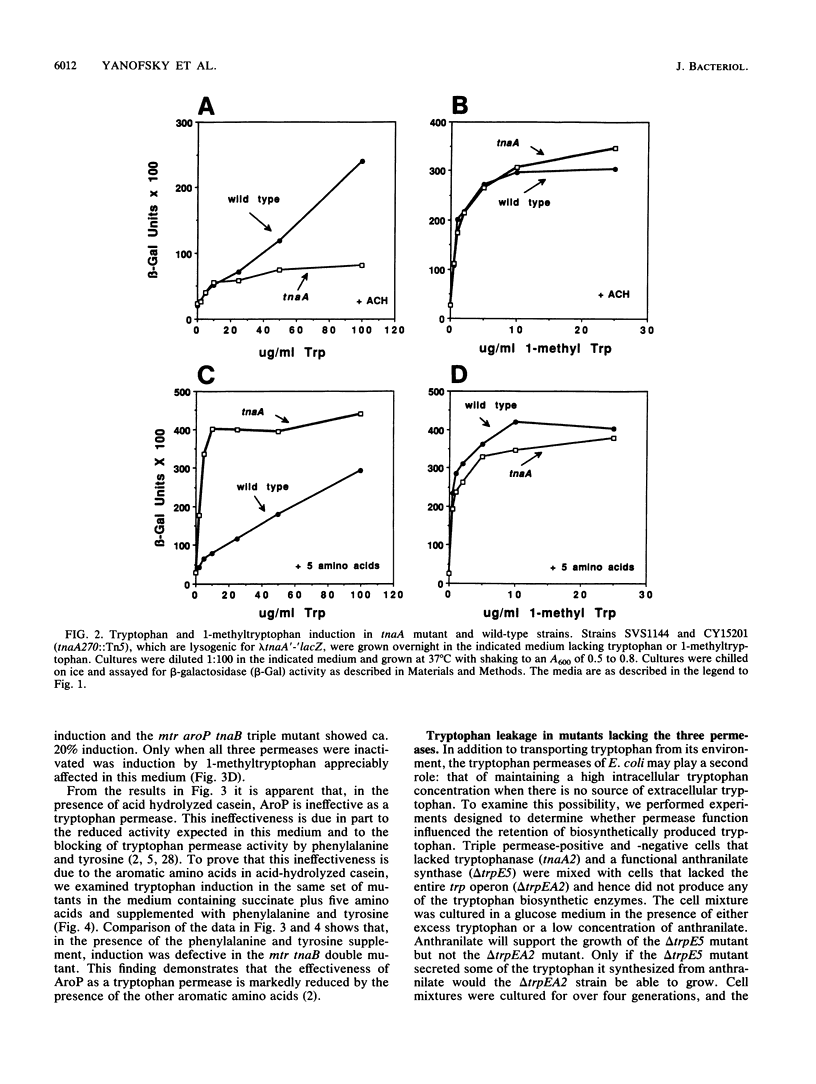
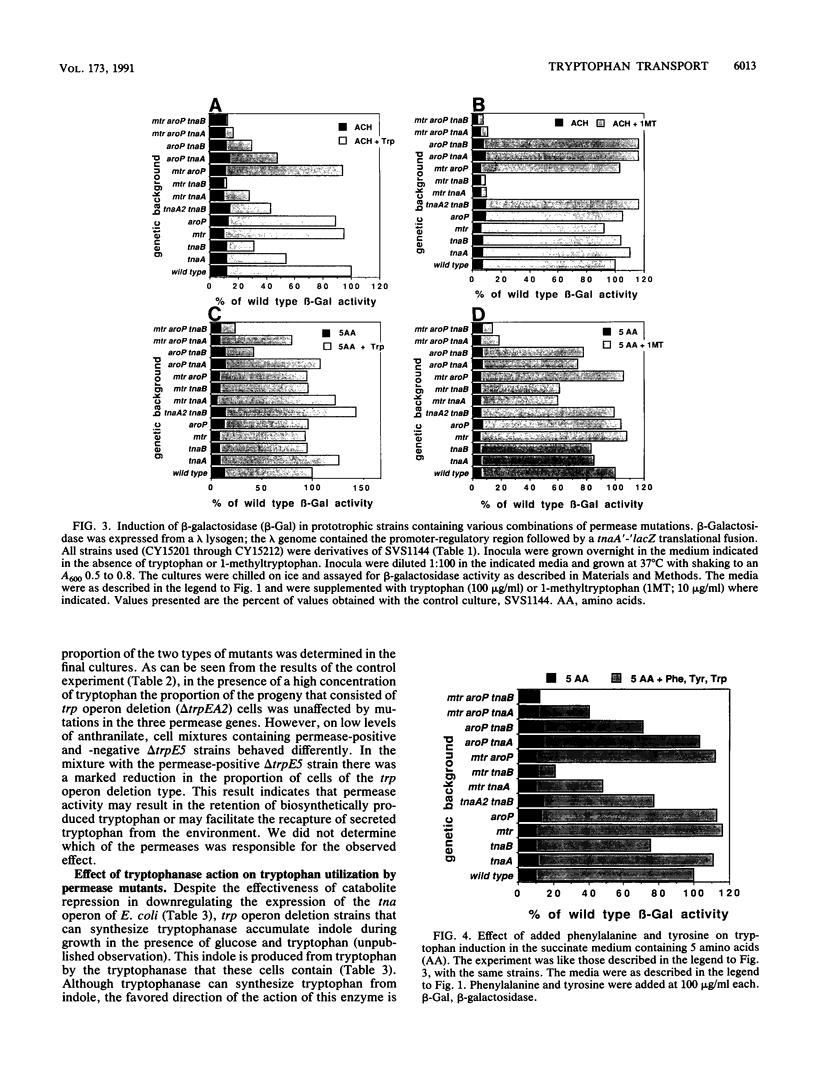
Escherichia coli forms three permeases that can transport the amino acid tryptophan: Mtr, AroP, and TnaB. The structural genes for these permeases reside in separate operons that are subject to different mechanisms of regulation. We have exploited the fact that the tryptophanase (tna) operon is induced by tryptophan to infer how tryptophan transport is influenced by the growth medium and by mutations that inactivate each of the permease proteins. In an acid-hydrolyzed casein medium, high levels of tryptophan are ordinarily required to obtain maximum tna operon induction. High levels are necessary because much of the added tryptophan is degraded by tryptophanase. An alternate inducer that is poorly cleaved by tryptophanase, 1-methyltryptophan, induces efficiently at low concentrations in both tna+ strains and tna mutants. In an acid-hydrolyzed casein medium, the TnaB permease is most critical for tryptophan uptake; i.e., only mutations in tnaB reduce tryptophanase induction. However, when 1-methyltryptophan replaces tryptophan as the inducer in this medium, mutations in both mtr and tnaB are required to prevent maximum induction. In this medium, AroP does not contribute to tryptophan uptake. However, in a medium lacking phenylalanine and tyrosine the AroP permease is active in tryptophan transport; under these conditions it is necessary to inactivate the three permeases to eliminate tna operon induction. The Mtr permease is principally responsible for transporting indole, the degradation product of tryptophan produced by tryptophanase action. The TnaB permease is essential for growth on tryptophan as the sole carbon source. When cells with high levels of tryptophanase are transferred to tryptophan-free growth medium, the expression of the tryptophan (trp) operon is elevated. This observation suggests that the tryptophanase present in these cells degrades some of the synthesized tryptophan, thereby creating a mild tryptophan deficiency. Our studies assign roles to the three permeases in tryptophan transport under different physiological conditions.
Full text
PDF

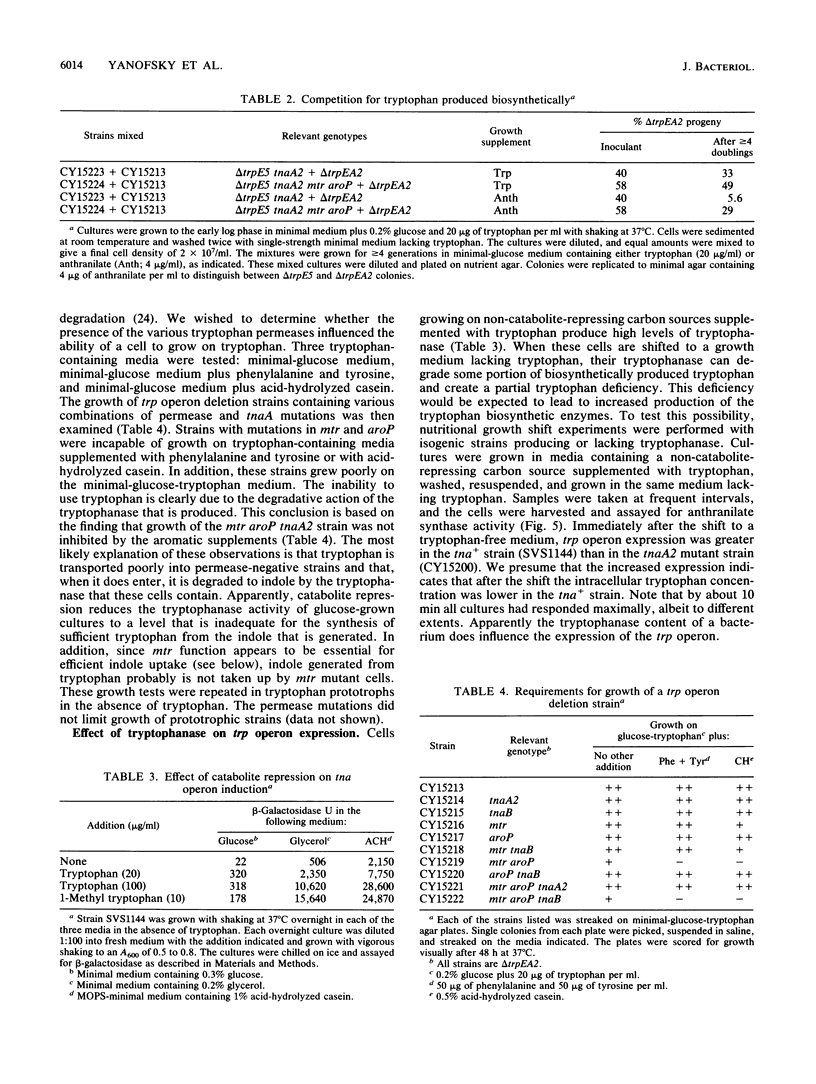
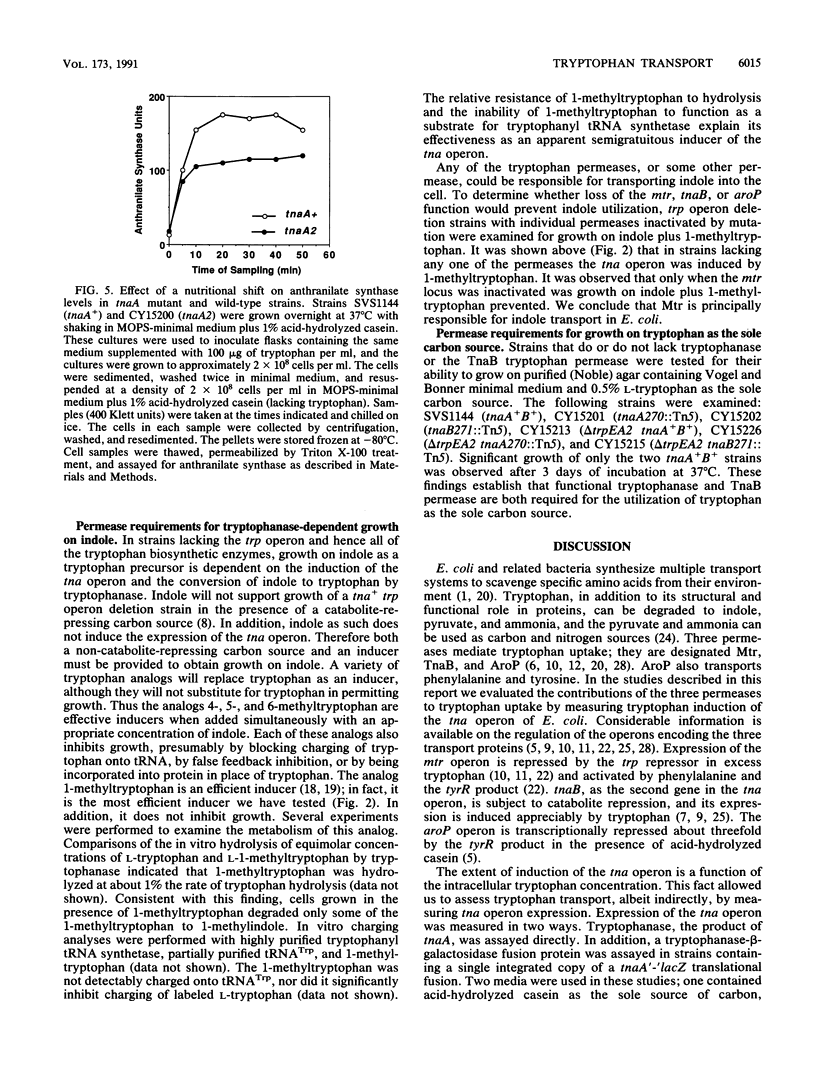


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Mimura C. S., Shyamala V. Bacterial periplasmic permeases belong to a family of transport proteins operating from Escherichia coli to human: Traffic ATPases. FEMS Microbiol Rev. 1990 Aug;6(4):429–446. doi: 10.1111/j.1574-6968.1990.tb04110.x. [DOI] [PubMed] [Google Scholar]
- BURROUS S. E., DEMOSS R. D. STUDIES ON TRYPTOPHAN PERMEASE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Aug 6;73:623–637. doi: 10.1016/0006-3002(63)90332-9. [DOI] [PubMed] [Google Scholar]
- Brown K. D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chye M. L., Guest J. R., Pittard J. Cloning of the aroP gene and identification of its product in Escherichia coli K-12. J Bacteriol. 1986 Aug;167(2):749–753. doi: 10.1128/jb.167.2.749-753.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chye M. L., Pittard J. Transcription control of the aroP gene in Escherichia coli K-12: analysis of operator mutants. J Bacteriol. 1987 Jan;169(1):386–393. doi: 10.1128/jb.169.1.386-393.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeley M. C., Yanofsky C. Nucleotide sequence of the structural gene for tryptophanase of Escherichia coli K-12. J Bacteriol. 1981 Sep;147(3):787–796. doi: 10.1128/jb.147.3.787-796.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. M., Yudkin M. D. Location of the gene for the low-affinity tryptophan-specific permease of Escherichia coli. Biochem J. 1982 May 15;204(2):617–619. doi: 10.1042/bj2040617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. M., Yudkin M. D. Tryptophanase synthesis in Escherichia coli: the role of indole replacement in supplying tryptophan and the nature of the constitutive mutation tnaR3. J Gen Microbiol. 1984 Jun;130(6):1535–1542. doi: 10.1099/00221287-130-6-1535. [DOI] [PubMed] [Google Scholar]
- Gollnick P., Yanofsky C. tRNA(Trp) translation of leader peptide codon 12 and other factors that regulate expression of the tryptophanase operon. J Bacteriol. 1990 Jun;172(6):3100–3107. doi: 10.1128/jb.172.6.3100-3107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatwole V. M., Somerville R. L. Cloning, nucleotide sequence, and characterization of mtr, the structural gene for a tryptophan-specific permease of Escherichia coli K-12. J Bacteriol. 1991 Jan;173(1):108–115. doi: 10.1128/jb.173.1.108-115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatwole V. M., Somerville R. L. The tryptophan-specific permease gene, mtr, is differentially regulated by the tryptophan and tyrosine repressors in Escherichia coli K-12. J Bacteriol. 1991 Jun;173(11):3601–3604. doi: 10.1128/jb.173.11.3601-3604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Ito K., Matsuyama T., Ozaki H., Yura T. 5-methyltryptophan-resistant mutations lniked with the arginine G marker in Escherichia coli. J Bacteriol. 1968 Nov;96(5):1880–1881. doi: 10.1128/jb.96.5.1880-1881.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré N., Cole S. T. Nucleotide sequence of the aroP gene encoding the general aromatic amino acid transport protein of Escherichia coli K-12: homology with yeast transport proteins. Nucleic Acids Res. 1990 Feb 11;18(3):653–653. doi: 10.1093/nar/18.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter R., Yanofsky C. Genetic analysis of the tryptophan operon regulatory region using site-directed mutagenesis. J Mol Biol. 1984 May 25;175(3):299–312. doi: 10.1016/0022-2836(84)90350-4. [DOI] [PubMed] [Google Scholar]
- Marmorstein R. Q., Sigler P. B. Stereochemical effects of L-tryptophan and its analogues on trp repressor's affinity for operator-DNA. J Biol Chem. 1989 Jun 5;264(16):9149–9154. [PubMed] [Google Scholar]
- Phillips R. S., Bender S. L., Brzovic P., Dunn M. F. Mechanism of binding of substrate analogues to tryptophan indole-lyase: studies using rapid-scanning and single-wavelength stopped-flow spectrophotometry. Biochemistry. 1990 Sep 18;29(37):8608–8614. doi: 10.1021/bi00489a016. [DOI] [PubMed] [Google Scholar]
- Phillips R. S., Gollnick P. D. Evidence that cysteine 298 is in the active site of tryptophan indole-lyase. J Biol Chem. 1989 Jun 25;264(18):10627–10632. [PubMed] [Google Scholar]
- Sarsero J. P., Wookey P. J., Gollnick P., Yanofsky C., Pittard A. J. A new family of integral membrane proteins involved in transport of aromatic amino acids in Escherichia coli. J Bacteriol. 1991 May;173(10):3231–3234. doi: 10.1128/jb.173.10.3231-3234.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsero J. P., Wookey P. J., Pittard A. J. Regulation of expression of the Escherichia coli K-12 mtr gene by TyrR protein and Trp repressor. J Bacteriol. 1991 Jul;173(13):4133–4143. doi: 10.1128/jb.173.13.4133-4143.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Snell E. E. Tryptophanase: structure, catalytic activities, and mechanism of action. Adv Enzymol Relat Areas Mol Biol. 1975;42:287–333. doi: 10.1002/9780470122877.ch6. [DOI] [PubMed] [Google Scholar]
- Stewart V., Yanofsky C. Evidence for transcription antitermination control of tryptophanase operon expression in Escherichia coli K-12. J Bacteriol. 1985 Nov;164(2):731–740. doi: 10.1128/jb.164.2.731-740.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suelter C. H., Wang J., Snell E. E. Direct spectrophotometric assay of tryptophanase. FEBS Lett. 1976 Jul 15;66(2):230–232. doi: 10.1016/0014-5793(76)80510-8. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Whipp M. J., Pittard A. J. Regulation of aromatic amino acid transport systems in Escherichia coli K-12. J Bacteriol. 1977 Nov;132(2):453–461. doi: 10.1128/jb.132.2.453-461.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]