Abstract
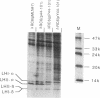
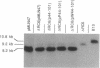
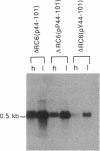
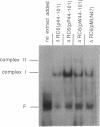
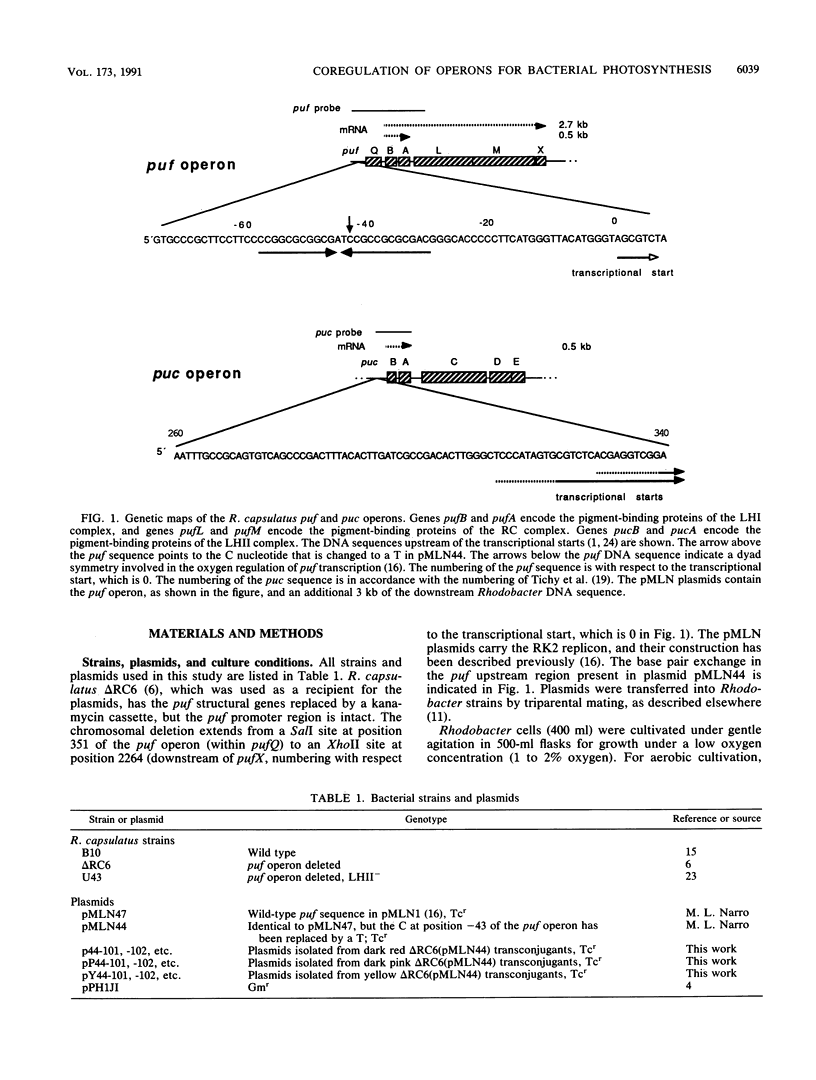
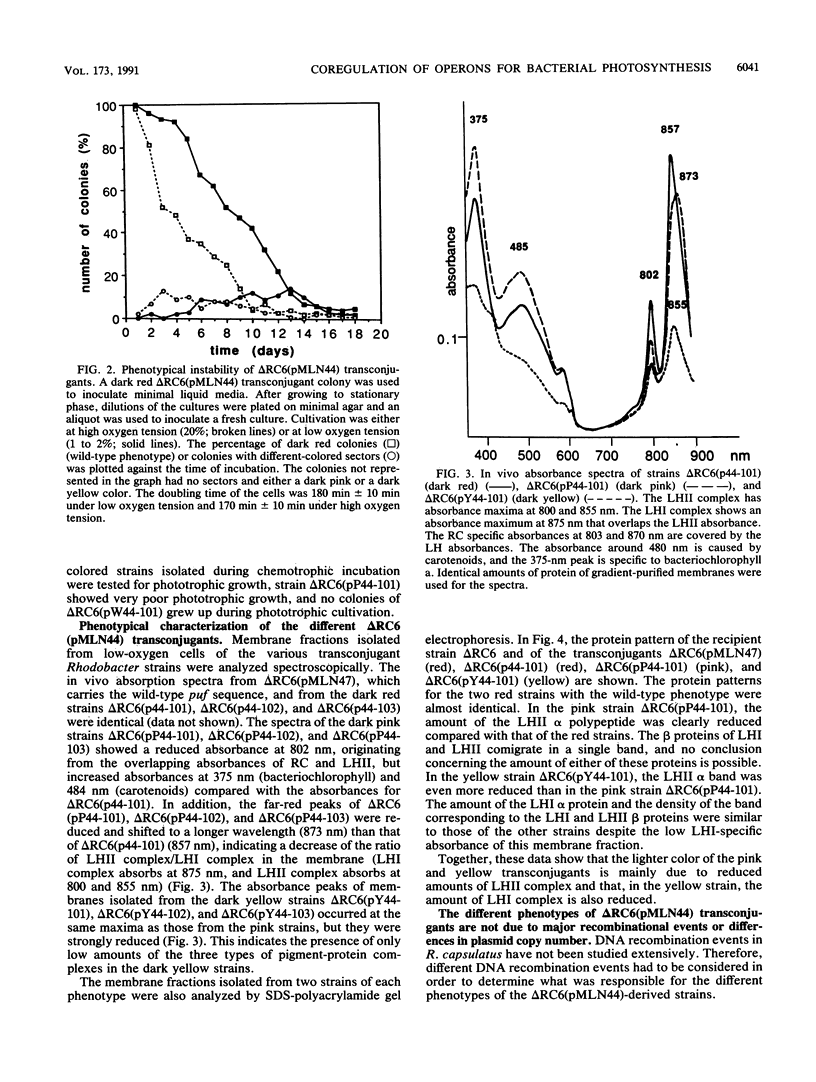
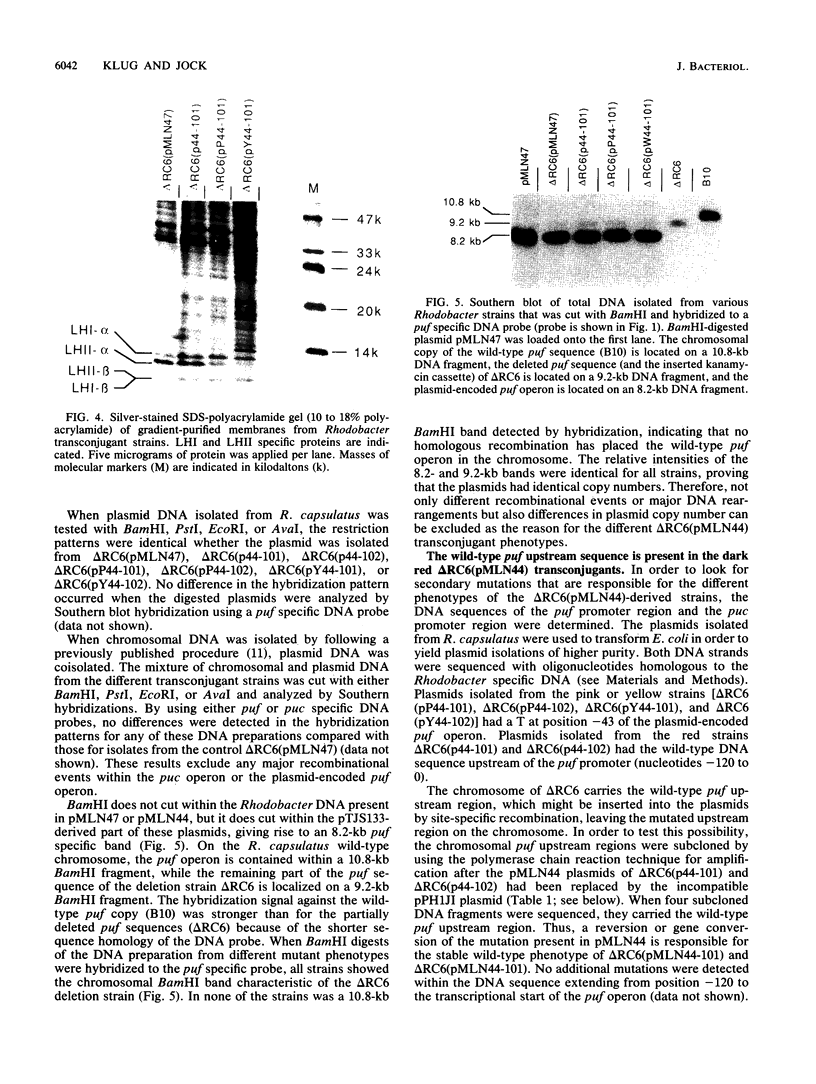
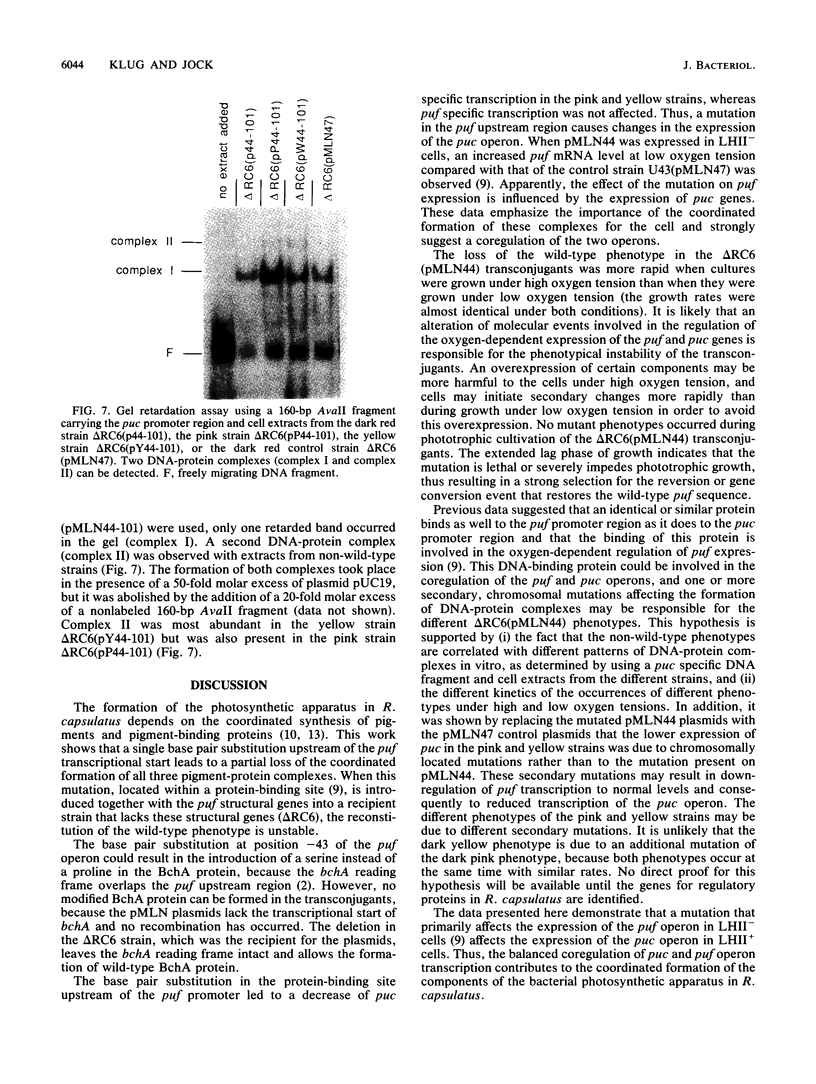
A DNA sequence with dyad symmetry upstream of the transcriptional start of the Rhodobacter capsulatus puf operon, which encodes pigment-binding proteins of the light-harvesting I complex and of the reaction center, has previously been shown to be a protein-binding site (G. Klug, Mol. Gen. Genet. 226:167-176, 1991). When a low-copy-number plasmid with a base pair transition at position -43 within this dyad symmetry in front of the puf structural genes was transferred into a Rhodobacter strain with the puf operon deleted, different phenotypes occurred during cultivation of the transconjugants and the kinetics of the loss of the wild-type phenotype was dependent on the oxygen tension in the culture. After growth for 150 generations, the different phenotypes were stably inherited. The strains having the wild-type phenotype carried the wild-type puf DNA sequence. The original mutation was still present in the strains that showed lighter color. These strains had less light-harvesting II complex in the membrane and showed lower rates of transcription of the puc operon, which encodes the proteins of this complex. This deregulation of puc expression was due to one or more chromosomally located, secondary mutations, not directly to the mutation present on the plasmid. Thus, a single-base-pair transition in the puf upstream region can result in a deregulation of puc expression, suggesting a direct or indirect transcriptional coregulation of both these operons by a common factor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Forrest M. E., Cohen S. N., Beatty J. T. Structural and functional analysis of transcriptional control of the Rhodobacter capsulatus puf operon. J Bacteriol. 1989 Jan;171(1):473–482. doi: 10.1128/jb.171.1.473-482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C. E., Young D. A., Marrs B. L. Analysis of the Rhodobacter capsulatus puf operon. Location of the oxygen-regulated promoter region and the identification of an additional puf-encoded gene. J Biol Chem. 1988 Apr 5;263(10):4820–4827. [PubMed] [Google Scholar]
- Belasco J. G., Beatty J. T., Adams C. W., von Gabain A., Cohen S. N. Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell. 1985 Jan;40(1):171–181. doi: 10.1016/0092-8674(85)90320-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. G., Davidson E., Marrs B. L. Variation of levels of mRNA coding for antenna and reaction center polypeptides in Rhodopseudomonas capsulata in response to changes in oxygen concentration. J Bacteriol. 1984 Mar;157(3):945–948. doi: 10.1128/jb.157.3.945-948.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G. A DNA sequence upstream of the puf operon of Rhodobacter capsulatus is involved in its oxygen-dependent regulation and functions as a protein binding site. Mol Gen Genet. 1991 Apr;226(1-2):167–176. doi: 10.1007/BF00273600. [DOI] [PubMed] [Google Scholar]
- Klug G., Cohen S. N. Pleiotropic effects of localized Rhodobacter capsulatus puf operon deletions on production of light-absorbing pigment-protein complexes. J Bacteriol. 1988 Dec;170(12):5814–5821. doi: 10.1128/jb.170.12.5814-5821.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G., Drews G. Construction of a gene bank of Rhodopseudomonas capsulata using a broad host range DNA cloning system. Arch Microbiol. 1984 Nov;139(4):319–325. doi: 10.1007/BF00408373. [DOI] [PubMed] [Google Scholar]
- Klug G., Kaufmann N., Drews G. Gene expression of pigment-binding proteins of the bacterial photosynthetic apparatus: Transcription and assembly in the membrane of Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6485–6489. doi: 10.1073/pnas.82.19.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marrs B. Genetic recombination in Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1974 Mar;71(3):971–973. doi: 10.1073/pnas.71.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narro M. L., Adams C. W., Cohen S. N. Isolation and characterization of Rhodobacter capsulatus mutants defective in oxygen regulation of the puf operon. J Bacteriol. 1990 Aug;172(8):4549–4554. doi: 10.1128/jb.172.8.4549-4554.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suissa M. Spectrophotometric quantitation of silver grains eluted from autoradiograms. Anal Biochem. 1983 Sep;133(2):511–514. doi: 10.1016/0003-2697(83)90117-3. [DOI] [PubMed] [Google Scholar]
- Tichy H. V., Oberlé B., Stiehle H., Schiltz E., Drews G. Genes downstream from pucB and pucA are essential for formation of the B800-850 complex of Rhodobacter capsulatus. J Bacteriol. 1989 Sep;171(9):4914–4922. doi: 10.1128/jb.171.9.4914-4922.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Ismail S., Bylina E. J. Chromosomal deletion and plasmid complementation of the photosynthetic reaction center and light-harvesting genes from Rhodopseudomonas capsulata. Gene. 1985;38(1-3):19–30. doi: 10.1016/0378-1119(85)90199-4. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Ismail S. Light-harvesting II (B800-B850 complex) structural genes from Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1985 Jan;82(1):58–62. doi: 10.1073/pnas.82.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucconi A. P., Beatty J. T. Posttranscriptional regulation by light of the steady-state levels of mature B800-850 light-harvesting complexes in Rhodobacter capsulatus. J Bacteriol. 1988 Feb;170(2):877–882. doi: 10.1128/jb.170.2.877-882.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]