Abstract
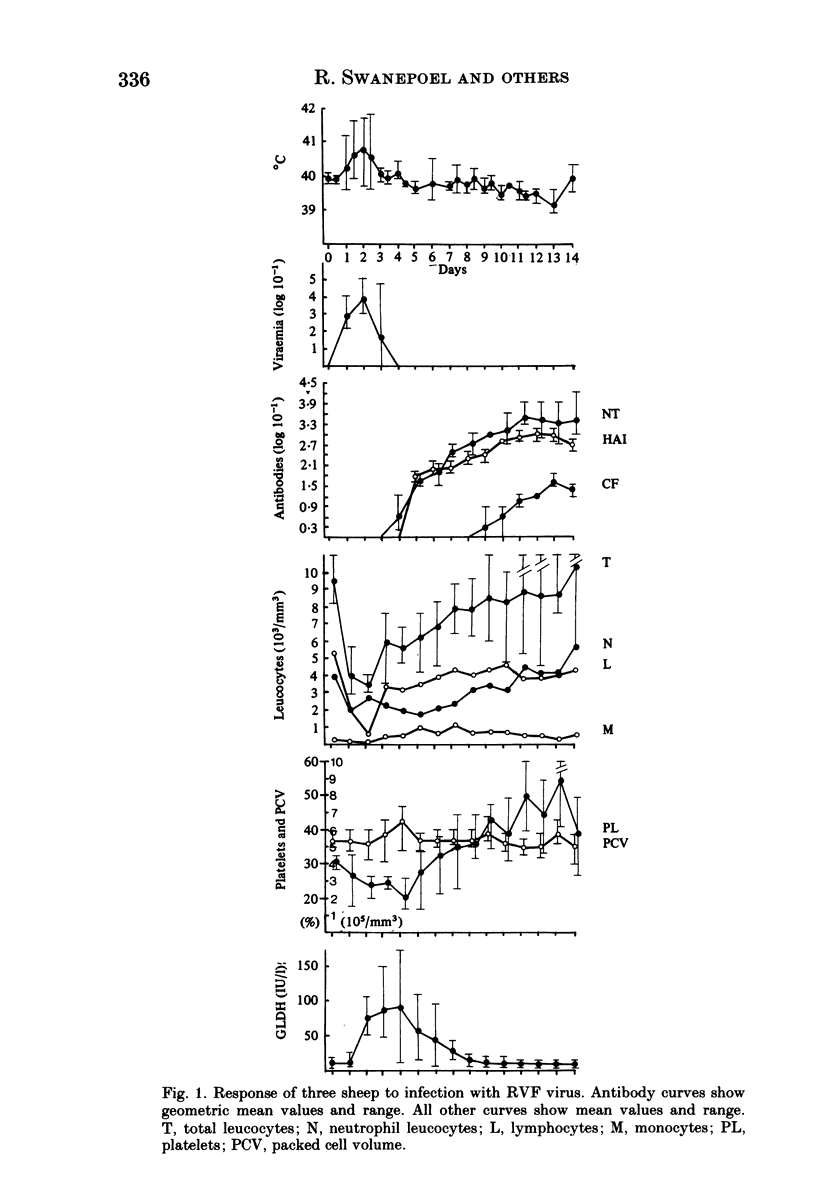
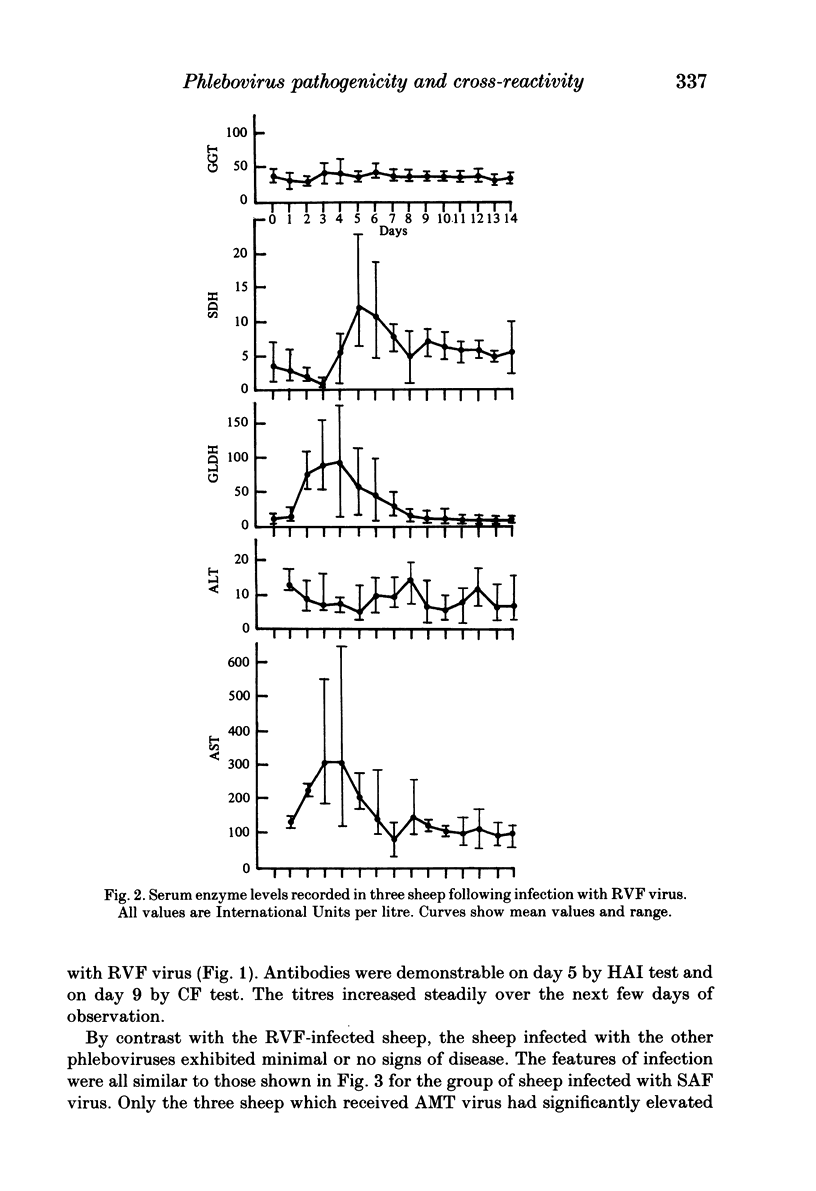
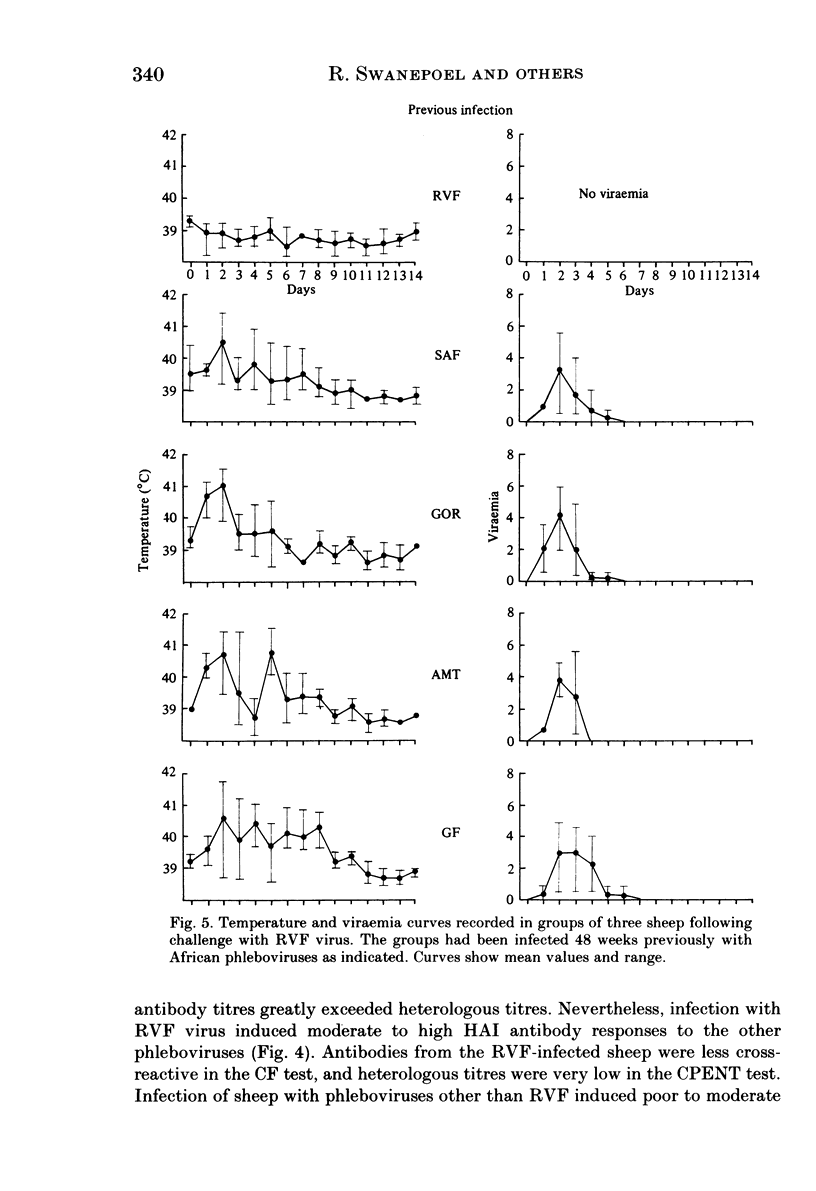
Homologous and heterologous haemagglutination-inhibition (HAI), complement-fixation (CF), immunodiffusion (ID) and mouse neutralization tests were performed with the Lunyo (LUN) and a Zimbabwean strain of Rift Valley fever (RVF) virus, the prototype and a South African strain of Arumowot (AMT) virus and prototype strains of Gordil (GOR), Saint-Floris (SAF) and Gabek Forest (GF) viruses, using immune mouse ascitic fluids prepared against these viruses. Reactions of identity occurred in all tests between LUN and the Zimbabwean strains of RVF and between the two strains of AMT virus. Otherwise, cross-reactions occurred between all the phleboviruses in HAI tests, while reactions in CF, ID and neutralization tests were monospecific for virus serotypes, except that weak cross-reaction occurred between GOR and SAF viruses in CF and ID tests. Four sheep infected subcutaneously with the Zimbabwean strain of RVF virus developed transient fever, viraemia, leucopaenia, relative thrombocytopaenia, haemoconcentration and raised serum enzyme levels, which indicated that the sheep had developed necrotic hepatitis. Disseminated focal necrotic hepatitis was confirmed in a sheep killed for examination on day 4 post-infection. The other three sheep recovered uneventfully after only mild depression and anorexia. Groups of three sheep infected with SAF, GOR, AMT and GF viruses had no demonstrable viraemia or other sign of infection or illness, except that the sheep infected with AMT developed mild fever lasting less than 24 h. Antibody responses were monitored at intervals over a period of 24 weeks in all sheep by homologous and heterologous HAI, CF and cell culture neutralization (CPENT) tests. Homologous antibody responses were marked in the RVF-infected sheep and their sera cross-reacted strongly in HAI tests with antigens of the other viruses. The sera of the RVF-infected sheep cross-reacted less markedly in CF and CPENT tests. Homologous antibody responses were poor in all the sheep infected with phleboviruses other than RVF, and the cross-reactivity of their sera for RVF antigen or virus was negligible. All sheep were challenged with RVF virus 48 weeks after their initial infection. The sheep which had originally been infected with RVF virus were immune and developed neither fever nor viraemia. All other sheep developed fever, viraemia and antibodies to RVF virus. It was concluded that the African phleboviruses, other than RVF, are unlikely to cause disease in livestock or to induce antibodies which could cause confusion in the diagnosis of RVF.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer R. K., Allen B. V., Baldwin C. A modified sedimentation method for counting platelets in blood. Br J Haematol. 1978 Mar;38(3):401–405. doi: 10.1111/j.1365-2141.1978.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Davies F. G. Observations on the epidemiology of Rift Valley fever in Kenya. J Hyg (Lond) 1975 Oct;75(2):219–230. doi: 10.1017/s0022172400047252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J., Bishop D. H. Recombination and complementation between temperature-sensitive mutants of a Bunyavirus, snowshoe hare virus. J Virol. 1976 Oct;20(1):351–354. doi: 10.1128/jvi.20.1.351-354.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimas R. A., Thompson W. H., Calisher C. H., Clark G. G., Grimstad P. R., Bishop D. H. Genotypic varieties of La Crosse virus isolated from different geographic regions of the continental United States and evidence for a naturally occurring intertypic recombinant La Crosse virus. Am J Epidemiol. 1981 Jul;114(1):112–131. doi: 10.1093/oxfordjournals.aje.a113158. [DOI] [PubMed] [Google Scholar]
- Meegan J. M., Digoutte J. P., Peters C. J., Shope R. E. Monoclonal antibodies to identify Zinga virus as Rift Valley Fever virus. Lancet. 1983 Mar 19;1(8325):641–641. doi: 10.1016/s0140-6736(83)91807-x. [DOI] [PubMed] [Google Scholar]
- Sartorelli A. C., Fischer D. S., Downs W. G. Use of sarcoma 180/TG to prepare hyperimmune ascitic fluid in the mouse. J Immunol. 1966 Apr;96(4):676–682. [PubMed] [Google Scholar]
- Shope R. E., Peters C. J., Walker J. S. Serological relation between Rift Valley fever virus and viruses of phlebotomus fever serogroup. Lancet. 1980 Apr 19;1(8173):886–887. doi: 10.1016/s0140-6736(80)91395-1. [DOI] [PubMed] [Google Scholar]
- Shope R. E. Seriologic and vector comparisons of Rift Valley virus with other bunyaviruses. J Egypt Public Health Assoc. 1978;53(3-4):235–242. [PubMed] [Google Scholar]
- Swanepoel R., Struthers J. K., Erasmus M. J., Shepherd S. P., McGillivray G. M., Erasmus B. J., Barnard B. J. Comparison of techniques for demonstrating antibodies to Rift Valley fever virus. J Hyg (Lond) 1986 Oct;97(2):317–329. doi: 10.1017/s0022172400065414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanepoel R. Studies on the epidemiology of Rift Valley Fever. J S Afr Vet Assoc. 1976 Jun;47(2):93–94. [PubMed] [Google Scholar]
- Tesh R. B., Peters C. J., Meegan J. M. Studies on the antigenic relationship among phleboviruses. Am J Trop Med Hyg. 1982 Jan;31(1):149–155. doi: 10.4269/ajtmh.1982.31.149. [DOI] [PubMed] [Google Scholar]
- Tomori O. Clinical, virological and serological response of the West African dwarf sheep to experimental infection with different strains of Rift Valley fever virus. Res Vet Sci. 1979 Mar;26(2):152–159. [PubMed] [Google Scholar]
- Tomori O. Immunological reactions of Rift Valley fever virus strains from East and West Africa. Res Vet Sci. 1979 Mar;26(2):160–164. [PubMed] [Google Scholar]
- Travassos da Rosa A. P., Tesh R. B., Pinheiro F. P., Travassos da Rosa J. F., Peterson N. E. Characterization of eight new phlebotomus fever serogroup arboviruses (Bunyaviridae: Phlebovirus) from the Amazon region of Brazil. Am J Trop Med Hyg. 1983 Sep;32(5):1164–1171. doi: 10.4269/ajtmh.1983.32.1164. [DOI] [PubMed] [Google Scholar]
- Ushijima H., Clerx-van Haaster M., Bishop D. H. Analyses of Patois group Bunyaviruses: evidence for naturally occurring recombinant Bunyaviruses and existence of immune precipitable and nonprecipitable nonvirion proteins induced in Burnyavirus-infected cells. Virology. 1981 Apr 30;110(2):318–332. [PubMed] [Google Scholar]
- WEINBREN M. P., WILLIAMS M. C., HADDOW A. J. A variant of Rift Valley fever virus. S Afr Med J. 1957 Sep 21;31(38):951–957. [PubMed] [Google Scholar]


