Abstract
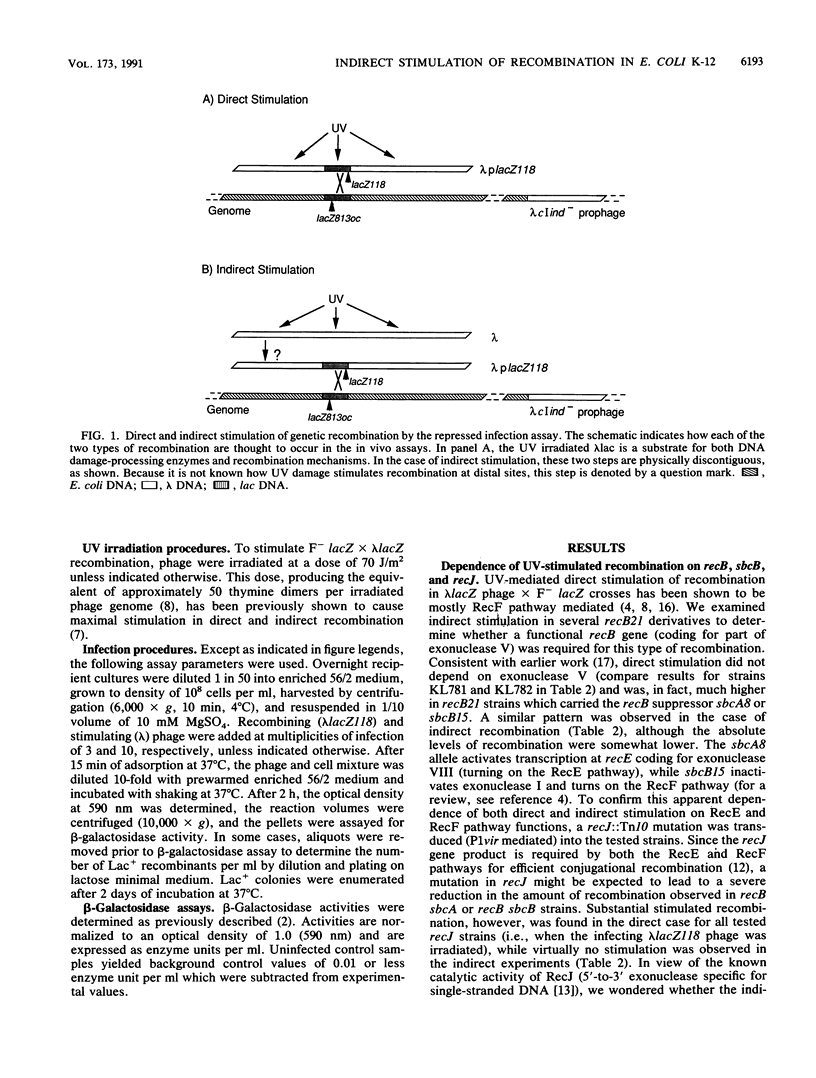
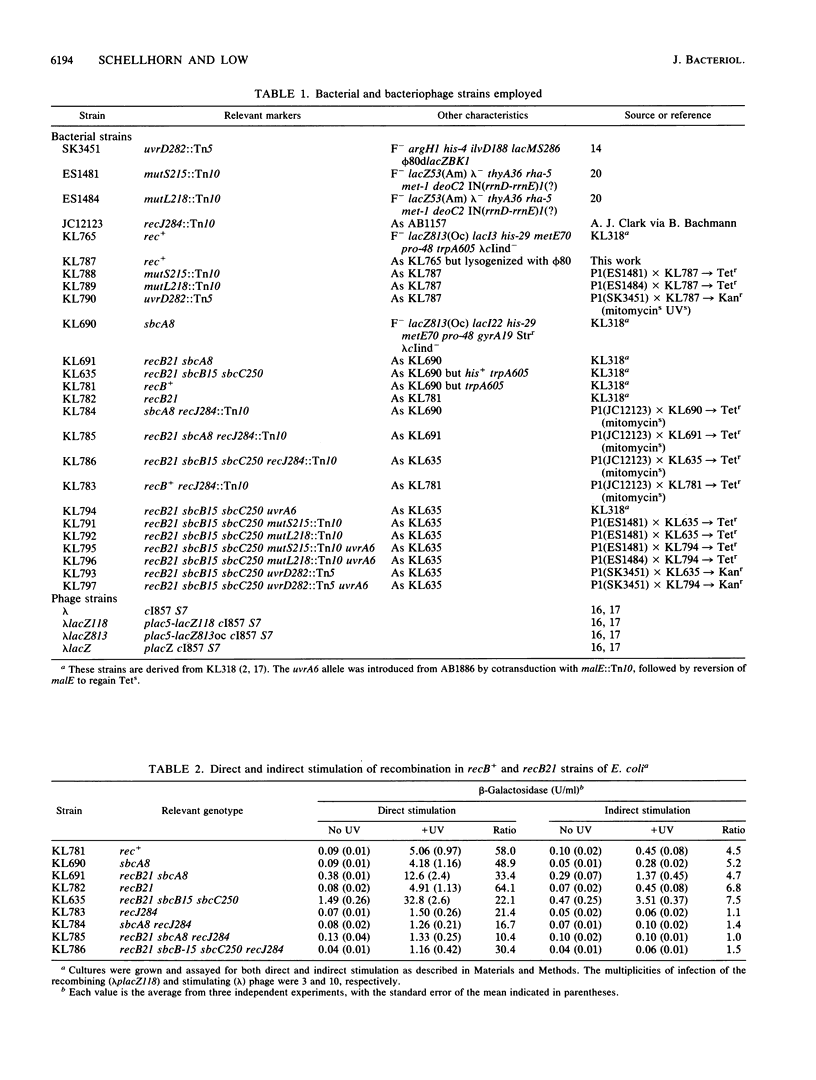
Direct and indirect UV-stimulated homologous genetic recombination was investigated in Escherichia coli strains blocked in several host-encoded functions. Genetic recombination was assayed by measuring beta-galactosidase produced after recombination between two noncomplementing lacZ ochre alleles. Both types of stimulation (direct and indirect) were found to be primarily RecF pathway-mediated. In a rec+ background, both direct and indirect stimulation were found to be dependent on uvrD (coding for helicase II). In a recB21 sbcB15 background, direct and indirect stimulation were uvrD dependent only when the strain was additionally deficient in the UvrABC excision repair pathway. Indirect but not direct stimulation was also dependent on recJ (coding for a 5'-to-3' exonuclease specific for single-stranded DNA) regardless of sbcA or sbcB configuration. The methyl-directed mismatch repair system (mutSLH) also appeared to play an important role in stimulation. On the basis of these findings, we suggest that excision of UV-induced DNA damage is a prelude to UV-mediated stimulation of genetic recombination.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboussekhra A., Chanet R., Zgaga Z., Cassier-Chauvat C., Heude M., Fabre F. RADH, a gene of Saccharomyces cerevisiae encoding a putative DNA helicase involved in DNA repair. Characteristics of radH mutants and sequence of the gene. Nucleic Acids Res. 1989 Sep 25;17(18):7211–7219. doi: 10.1093/nar/17.18.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge E. A., Low K. B. Detection of transcribable recombination products following conjugation in rec+, reCB- and recC-strains of Escherichia coli K12. J Mol Biol. 1974 Mar 15;83(4):447–457. doi: 10.1016/0022-2836(74)90506-3. [DOI] [PubMed] [Google Scholar]
- Caron P. R., Kushner S. R., Grossman L. Involvement of helicase II (uvrD gene product) and DNA polymerase I in excision mediated by the uvrABC protein complex. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4925–4929. doi: 10.1073/pnas.82.15.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta C., Radding C. M. Polar branch migration promoted by recA protein: effect of mismatched base pairs. Proc Natl Acad Sci U S A. 1982 Feb;79(3):762–766. doi: 10.1073/pnas.79.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein S. I., Low K. B. Hyper-recombining recipient strains in bacterial conjugation. Genetics. 1986 May;113(1):13–33. doi: 10.1093/genetics/113.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub E. I., Low K. B. Indirect stimulation of genetic recombination. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1401–1405. doi: 10.1073/pnas.80.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays J. B., Lee E. Repair and recombination of nonreplicating UV-irradiated phage DNA in E. coli III. Enhancement of excision repair in UV-treated bacteria. Mol Gen Genet. 1985;201(3):402–408. doi: 10.1007/BF00331330. [DOI] [PubMed] [Google Scholar]
- Hays J. B., Martin S. J., Bhatia K. Repair of nonreplicating UV-irradiated DNA: cooperative dark repair by Escherichia coli uvr and phr functions. J Bacteriol. 1985 Feb;161(2):602–608. doi: 10.1128/jb.161.2.602-608.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays J. B., Smith T. A., Friedman S. A., Lee E., Coffman G. L. RecF and RecBC function during recombination of nonreplicating, UV-irradiated phage lambda DNA and during other recombination processes. Cold Spring Harb Symp Quant Biol. 1984;49:475–483. doi: 10.1101/sqb.1984.049.01.054. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Fishel R. A., Howard M. Genetic recombination of bacterial plasmid DNA: effect of RecF pathway mutations on plasmid recombination in Escherichia coli. J Bacteriol. 1985 Sep;163(3):1060–1066. doi: 10.1128/jb.163.3.1060-1066.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett S. T., Clark A. J. Genetic analysis of the recJ gene of Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):190–196. doi: 10.1128/jb.157.1.190-196.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett S. T., Kolodner R. D. Identification and purification of a single-stranded-DNA-specific exonuclease encoded by the recJ gene of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2627–2631. doi: 10.1073/pnas.86.8.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maples V. F., Kushner S. R. DNA repair in Escherichia coli: identification of the uvrD gene product. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5616–5620. doi: 10.1073/pnas.79.18.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., McLaughlin T., Low B. Transduction versus "conjuduction": evidence for multiple roles for exonuclease V in genetic recombination in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1043–1047. doi: 10.1101/sqb.1979.043.01.113. [DOI] [PubMed] [Google Scholar]
- Porter R. D. Specialized transduction with lambda plac5: involvement of the RecE and RecF recombination pathways. Genetics. 1983 Oct;105(2):247–257. doi: 10.1093/genetics/105.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Machin N., Stahl F. W. A DNA double chain break stimulates triparental recombination in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6225–6229. doi: 10.1073/pnas.86.16.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyon G. T., Bear D. G., Lohman T. M. Escherichia coli helicase II (UvrD) protein initiates DNA unwinding at nicks and blunt ends. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6383–6387. doi: 10.1073/pnas.87.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. C., Wain S. L., Meltzer S. F., Binion M. L., Steinberg J. L. Mutator mutations in Escherichia coli induced by the insertion of phage mu and the transposable resistance elements Tn5 and Tn10. Mutat Res. 1982 Mar;93(1):25–33. doi: 10.1016/0027-5107(82)90122-1. [DOI] [PubMed] [Google Scholar]
- Smith T. A., Hays J. B. Repair and recombination of nonreplicating UV-irradiated phage DNA in E. coli II. Stimulation of RecF-dependent recombination by excision repair of cyclobutane pyrimidine dimers and of other photoproducts. Mol Gen Genet. 1985;201(3):393–401. doi: 10.1007/BF00331329. [DOI] [PubMed] [Google Scholar]
- Van Houten B. Nucleotide excision repair in Escherichia coli. Microbiol Rev. 1990 Mar;54(1):18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G., van Ham R. C., Vermeulen W., Bootsma D., van der Eb A. J., Hoeijmakers J. H. A presumed DNA helicase encoded by ERCC-3 is involved in the human repair disorders xeroderma pigmentosum and Cockayne's syndrome. Cell. 1990 Aug 24;62(4):777–791. doi: 10.1016/0092-8674(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Zieg J., Maples V. F., Kushner S. R. Recombinant levels of Escherichia coli K-12 mutants deficient in various replication, recombination, or repair genes. J Bacteriol. 1978 Jun;134(3):958–966. doi: 10.1128/jb.134.3.958-966.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


