Abstract
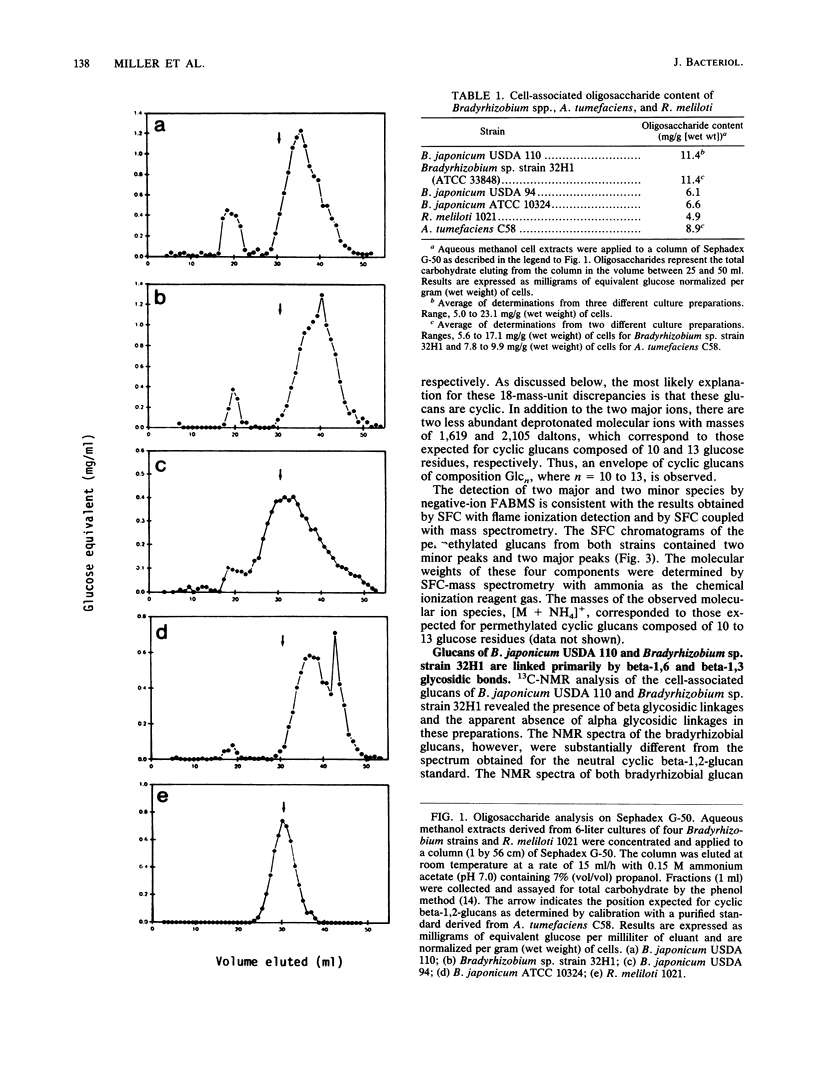
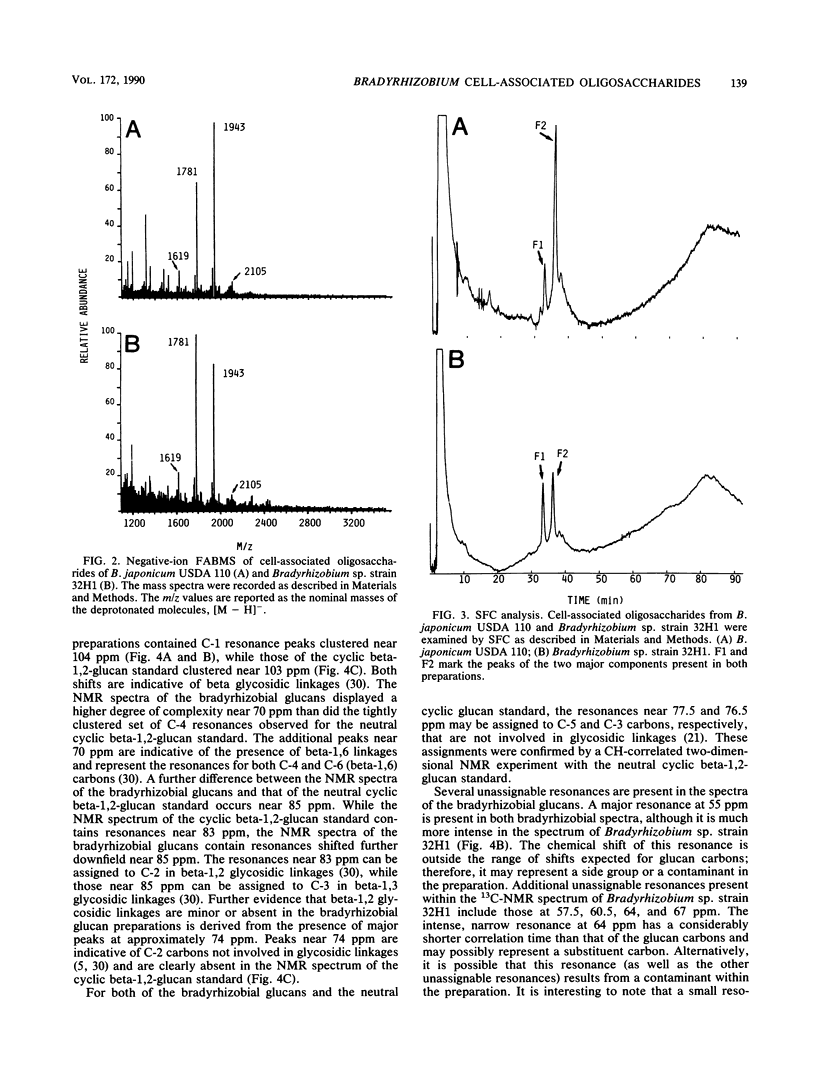
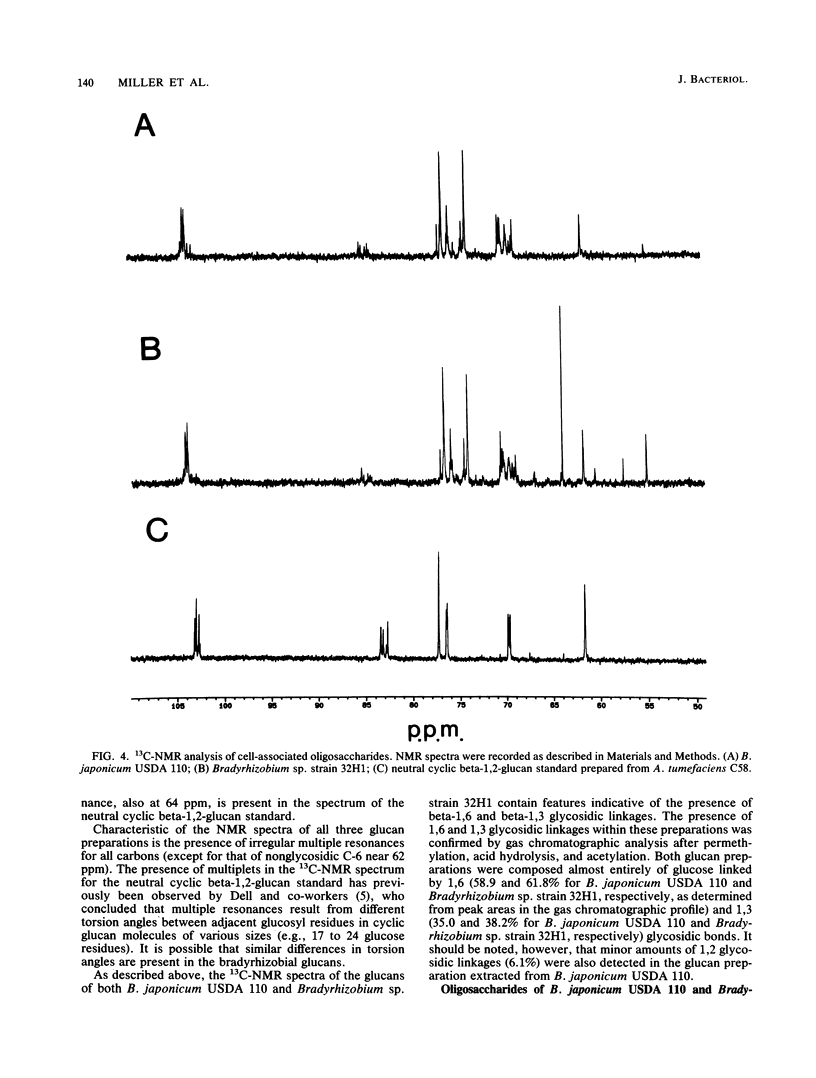
We report the initial characterization of the cell-associated oligosaccharides produced by four Bradyrhizobium strains: Bradyrhizobium japonicum USDA 110, USDA 94, and ATCC 10324 and Bradyrhizobium sp. strain 32H1. The cell-associated oligosaccharides of these strains were found to be composed solely of glucose and were predominantly smaller than the cyclic beta-1,2-glucans produced by Agrobacterium and Rhizobium species. Linkage studies and nuclear magnetic resonance analyses demonstrated that the bradyrhizobial glucans are linked primarily by beta-1,6 and beta-1,3 glycosidic bonds. Thus, the bradyrhizobia appear to synthesize cell-associated oligosaccharides of structural character substantially different from that of the cyclic beta-1,2-glucans produced by Agrobacterium and Rhizobium species.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DEDONDER R. A., HASSID W. Z. THE ENZYMATIC SYNTHESIS OF A (BETA-I,2-)-LINKED GLUCAN BY AN EXTRACT OF RHIZOBIUM JAPONICUM. Biochim Biophys Acta. 1964 Aug 19;90:239–248. doi: 10.1016/0304-4165(64)90187-4. [DOI] [PubMed] [Google Scholar]
- Dylan T., Ielpi L., Stanfield S., Kashyap L., Douglas C., Yanofsky M., Nester E., Helinski D. R., Ditta G. Rhizobium meliloti genes required for nodule development are related to chromosomal virulence genes in Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4403–4407. doi: 10.1073/pnas.83.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENCH D. The Schardinger dextrins. Adv Carbohydr Chem. 1957;12:189–260. doi: 10.1016/s0096-5332(08)60209-x. [DOI] [PubMed] [Google Scholar]
- Geremia R. A., Cavaignac S., Zorreguieta A., Toro N., Olivares J., Ugalde R. A. A Rhizobium meliloti mutant that forms ineffective pseudonodules in alfalfa produces exopolysaccharide but fails to form beta-(1----2) glucan. J Bacteriol. 1987 Feb;169(2):880–884. doi: 10.1128/jb.169.2.880-884.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Signal exchange in plant-microbe interactions. Microbiol Rev. 1986 Jun;50(2):193–225. doi: 10.1128/mr.50.2.193-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. J., Henry R. J., Blakeney A. B., Stone B. A. An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydr Res. 1984 Apr 2;127(1):59–73. doi: 10.1016/0008-6215(84)85106-x. [DOI] [PubMed] [Google Scholar]
- Kennedy L. D., Bailey R. W. Monomethyl sugars in extracellular polysaccharides from slow-growing Rhizobia. Carbohydr Res. 1976 Jul;49:451–454. doi: 10.1016/s0008-6215(00)83162-6. [DOI] [PubMed] [Google Scholar]
- Miller K. J., Gore R. S., Benesi A. J. Phosphoglycerol substituents present on the cyclic beta-1,2-glucans of Rhizobium meliloti 1021 are derived from phosphatidylglycerol. J Bacteriol. 1988 Oct;170(10):4569–4575. doi: 10.1128/jb.170.10.4569-4575.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P., Reinhold V. N. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986 Jan 3;231(4733):48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Miller K. J., Reinhold V. N., Weissborn A. C., Kennedy E. P. Cyclic glucans produced by Agrobacterium tumefaciens are substituted with sn-1-phosphoglycerol residues. Biochim Biophys Acta. 1987 Jul 10;901(1):112–118. doi: 10.1016/0005-2736(87)90262-8. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Application of two new methods for cleavage of polysaccharides into specific oligosaccharide fragments. Structure of the capsular and extracellular polysaccharides of Rhizobium japonicum that bind soybean lectin. J Biol Chem. 1982 Feb 25;257(4):1870–1875. [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Composition of the Capsular and Extracellular Polysaccharides of Rhizobium japonicum: CHANGES WITH CULTURE AGE AND CORRELATIONS WITH BINDING OF SOYBEAN SEED LECTIN TO THE BACTERIA . Plant Physiol. 1980 Jul;66(1):158–163. doi: 10.1104/pp.66.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Stacey G., Douglas C. J., Nester E. W. Role for 2-linked-beta-D-glucan in the virulence of Agrobacterium tumefaciens. J Bacteriol. 1985 Oct;164(1):102–106. doi: 10.1128/jb.164.1.102-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold V. N., Sheeley D. M., Kuei J., Her G. R. Analysis of high molecular weight samples on a double-focusing magnetic sector instrument by supercritical fluid chromatography/mass spectrometry. Anal Chem. 1988 Dec 15;60(24):2719–2722. doi: 10.1021/ac00175a016. [DOI] [PubMed] [Google Scholar]
- Ryan C. A. Oligosaccharide signalling in plants. Annu Rev Cell Biol. 1987;3:295–317. doi: 10.1146/annurev.cb.03.110187.001455. [DOI] [PubMed] [Google Scholar]


