Abstract
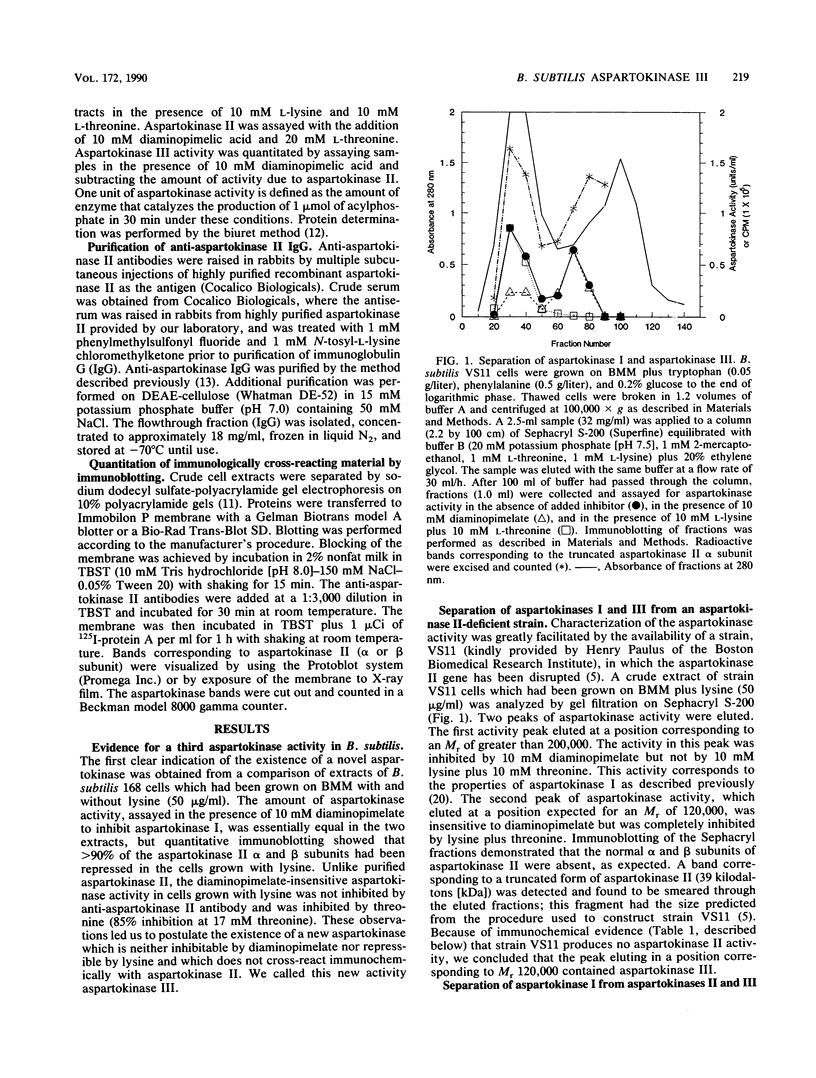
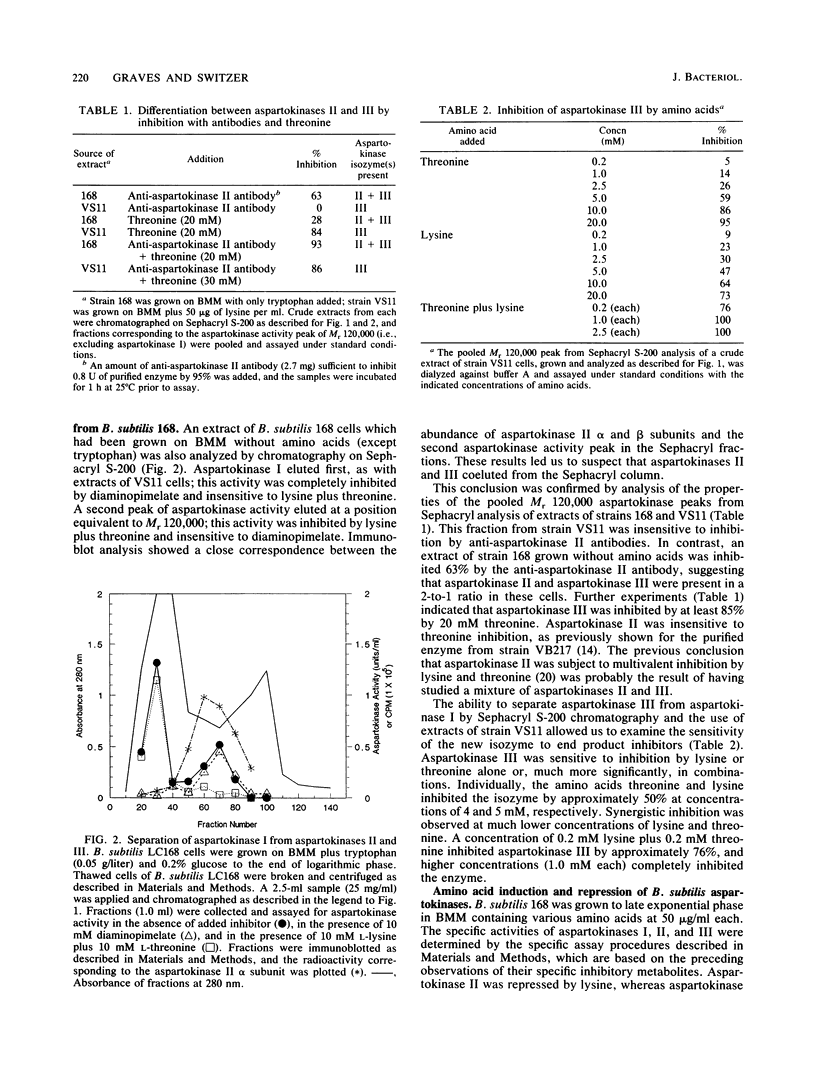
A previously undetected Bacillus subtilis aspartokinase isozyme, which we have called aspartokinase III, has been characterized. The new isozyme was most readily detected in extracts of cells grown with lysine, which repressed aspartokinase II and induced aspartokinase III, or in extracts of strain VS11, a mutant lacking aspartokinase II. Antibodies against aspartokinase II did not cross-react with aspartokinase III. Aspartokinases II and III coeluted on gel filtration chromatography at Mr 120,000, which accounts for the previous inability to detect it. Aspartokinase III was induced by lysine and repressed by threonine. It was synergistically inhibited by lysine and threonine. Aspartokinase III activity, like aspartokinase II activity, declined rapidly in B. subtilis cells that were starved for glucose. In contrast, the specific activity of aspartokinase I, the diaminopimelic acid-inhibitable isozyme, was constant under all growth conditions examined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernlohr D. A., Switzer R. L. Regulation of Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase inactivation in vivo. J Bacteriol. 1983 Feb;153(2):937–949. doi: 10.1128/jb.153.2.937-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas C., Gray E., Paulus H. Multivalent feedback inhibition of aspartokinase in Bacillus polymyxa. 3. Purification and subunit structure of the enzyme. J Biol Chem. 1970 Oct 10;245(19):4900–4906. [PubMed] [Google Scholar]
- Bondaryk R. P., Paulus H. Cloning and structure of the gene for the subunits of aspartokinase II from Bacillus subtilis. J Biol Chem. 1985 Jan 10;260(1):585–591. [PubMed] [Google Scholar]
- Bondaryk R. P., Paulus H. Expression of the gene for Bacillus subtilis aspartokinase II in Escherichia coli. J Biol Chem. 1985 Jan 10;260(1):592–597. [PubMed] [Google Scholar]
- Chen N. Y., Paulus H. Mechanism of expression of the overlapping genes of Bacillus subtilis aspartokinase II. J Biol Chem. 1988 Jul 5;263(19):9526–9532. [PubMed] [Google Scholar]
- Gray B. H., Bernlohr R. W. The regulation of aspartokinase in Bacillus licheniformis. Biochim Biophys Acta. 1969 Apr 22;178(2):248–261. doi: 10.1016/0005-2744(69)90394-5. [DOI] [PubMed] [Google Scholar]
- Hampton M. L., McCormick N. G., Behforouz N. C., Freese E. Regulation of two aspartokinases in Bacillus subtilis. J Bacteriol. 1971 Dec;108(3):1129–1134. doi: 10.1128/jb.108.3.1129-1134.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock M. H., Hodgson B. Lysine- and lysine-plus-threonine-inhibitable aspartokinases in Bacillus brevis. Biochim Biophys Acta. 1976 Sep 14;445(2):350–363. doi: 10.1016/0005-2744(76)90089-9. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maurizi M. R., Brabson J. S., Switzer R. L. Immunochemical studies of the inactivation of aspartate transcarbamylase by stationary phase Bacillus subtilis cells. Evidence for selective, energy-dependent degradation. J Biol Chem. 1978 Aug 25;253(16):5585–5593. [PubMed] [Google Scholar]
- Moir D., Paulus H. Properties and subunit structure of aspartokinase II from Bacillus subtilis VB217. J Biol Chem. 1977 Jul 10;252(13):4648–4651. [PubMed] [Google Scholar]
- Paulus H., Gray E. Multivalent feedback inhibition of aspartokinase in Bacillus polymyxa. I. Effect of nonpolar L-amino acids. J Biol Chem. 1968 Apr 10;243(7):1349–1355. [PubMed] [Google Scholar]
- Paulus H., Gray E. Multivalent feedback inhibition of aspartokinase in Bacillus polymyxa. I. Kinetic studies. J Biol Chem. 1967 Nov 10;242(21):4980–4986. [PubMed] [Google Scholar]
- Rosner A., Paulus H. Regulation of aspartokinase in Bacillus subtilis. The separation and properties of two isofunctional enzymes. J Biol Chem. 1971 May 10;246(9):2965–2971. [PubMed] [Google Scholar]
- Stadtman E. R. Allosteric regulation of enzyme activity. Adv Enzymol Relat Areas Mol Biol. 1966;28:41–154. doi: 10.1002/9780470122730.ch2. [DOI] [PubMed] [Google Scholar]
- Switzer R. L., Bond R. W., Ruppen M. E., Rosenzweig S. Involvement of the stringent response in regulation of protein degradation in Bacillus subtilis. Curr Top Cell Regul. 1985;27:373–386. doi: 10.1016/b978-0-12-152827-0.50039-6. [DOI] [PubMed] [Google Scholar]
- Switzer R. L., Gibson K. J. Phosphoribosylpyrophosphate synthetase (ribose-5-phosphate pyrophosphokinase) from Salmonella typhimurium. Methods Enzymol. 1978;51:3–11. doi: 10.1016/s0076-6879(78)51003-3. [DOI] [PubMed] [Google Scholar]
- Switzer R. L. The inactivation of microbial enzymes in vivo. Annu Rev Microbiol. 1977;31:135–157. doi: 10.1146/annurev.mi.31.100177.001031. [DOI] [PubMed] [Google Scholar]


