Abstract
Acute, inescapable, and unpredictable stress can profoundly modify brain and cognition in humans and animals. The present study investigated the ensuing effects of 2-h variable “audiogenic” stress on three related levels of hippocampal functions in rats: long-term potentiation, place cell activity, and spatial memory. In agreement with prior findings, we observed that stress reduced the magnitude of Schaffer collateral/commissural–Cornu Ammonis field 1 long-term potentiation in vitro, and selectively impaired spatial memory on a hidden platform version of the Morris water maze task. We also observed that stress impaired the stability of firing rates (but not firing locations) of place cells recorded from dorsal Cornu Ammonis field 1 in rats foraging freely on a novel open-field platform located in a familiar surrounding room. These findings suggest that stress-induced modifications in synaptic plasticity may prevent the storage of stable “rate maps” by hippocampal place cells, which in turn may contribute to spatial memory impairments associated with stress.
Keywords: consolidation, corticosterone, hippocampus, long-term potentiation, single unit
Stress is a biologically significant environmental factor that plays a pervasive role in humans and animals, from impacting daily behaviors to producing and exacerbating myriad diseases (1, 2). Recent studies reveal that while the acute, heightened cognitive response to stress is an adaptive mechanism, chronic and/or intense acute stress can consequently be the source of detrimental neurocognitive effects, particularly in the hippocampus (3–6).
The hippocampus is a medial temporal lobe structure implicated in the consolidation of declarative memory in humans and spatial memory in rodents (7–9). However, because it is concentrated with receptors for corticosteroids, the principal glucocorticoid hormones significantly elevated in response to stress (cortisol in human; corticosterone in rodent), the hippocampus is also quite responsive to stress (3–6). This responsiveness has implications for its role in cognition, as stress generally hinders later performance on hippocampal memory tasks in humans and animals (see ref. 6 for tasks enhanced by stress). For example, long-term verbal recall performance declines in healthy subjects exposed to stress (10, 11) and in posttraumatic stress disorder patients (12, 13). In rodents, stress produces deficits in spatial memory tasks (14–17).
Paralleling these behavioral findings are physiological studies indicating that stress blocks long-term potentiation (LTP) induction in the hippocampus (4–6). LTP is a prime candidate synaptic mechanism for information storage in the brain (18, 19). Originally, Thompson and colleagues (20) discovered that hippocampal slices prepared from rats exposed to 30 min of restraint plus intermittent tailshocks exhibited impaired LTP in the Cornu Ammonis field 1 (CA1) area. Subsequent studies found that LTP deficits are largely caused by psychological (and not pain) aspects of stress (21, 22) and depend on NMDA receptor (23) and amygdalar activities (17, 24). Additionally, stress effects have been demonstrated in anesthetized and awake animals (15, 25) and observed in the dentate gyrus (DG; ref. 26) and are contingent on a critical stress threshold (21) and transient (27). Notably, stress effects on LTP can occur without corticosterone (CORT) elevation (28), and normal LTP can be produced under significantly elevated CORT (17), suggesting that CORT elevation is not sufficient to reproduce stress effects (4). Because stress influences LTP and memory similarly, it has been postulated that stress-induced LTP impairments subserve stress-induced memory impediments (3–6).
Although stress effects on plasticity and memory have been well studied, much less is known about stress effects on hippocampal neuronal activities in behaving animals. The rodent hippocampus contains “place cells,” which are thought to support spatial learning/navigation by encoding memories of familiar spatial locations (8, 29, 30). Each place cell fires selectively when the animal visits a preferred location within a familiar environment, and each cell's preferred firing location remains stable across repeated visits to the same environment (31, 32). When a rat first visits a novel environment, place cells gradually establish their preferred firing locations within that environment over a period of minutes, and this formation of new place fields is thought to involve synaptic plasticity within the hippocampus, a view that is supported by studies showing that genetic and pharmacological manipulations that disrupt hippocampal LTP and spatial learning also disrupt the stability of hippocampal place cells (e.g., refs. 33–38). Because stress impairs hippocampal LTP and spatial memory, we investigated whether stress would also affect hippocampal place cells. To do so, we examined the effects of audiogenic stress (39, 40) on three related levels of hippocampal function (LTP, place cells, and spatial memory) in rats. We report that stress reduced LTP in vitro, altered place cell stability, and impeded the consolidation of spatial memory.
Results
Stress Effects on LTP.
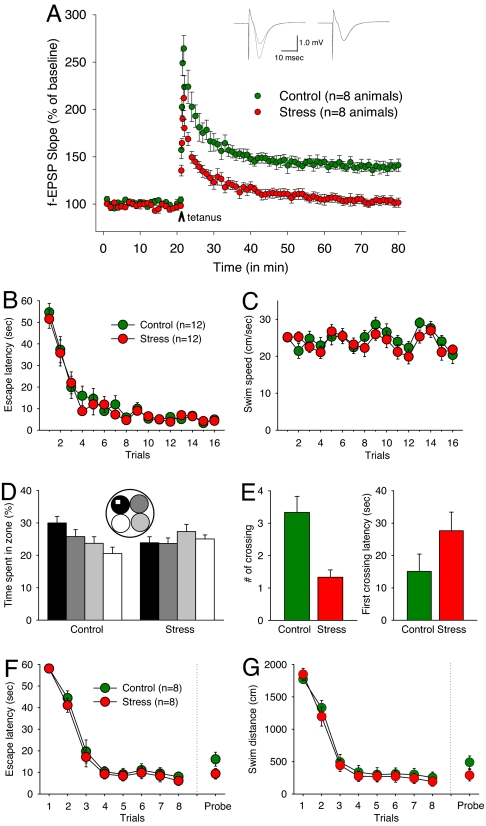
After an acute (2-h) exposure to audiogenic stress, hippocampal slices were prepared, and then LTP was assessed according to published protocol (ref. 41; see Materials and Methods). Slices from stressed rats exhibited markedly impaired LTP [normalized field excitatory postsynaptic potential (f-EPSP) slopes measured 40–60 min after the tetanus: 103.6 ± 4.4%], whereas LTP was reliable in slices from control rats (139.9 ± 5.6%). This observation was statistically supported by a significant main effect of group (Fig. 1A; P < 0.001, ANOVA). Thus, audiogenic stress effectively impaired ensuing induction of LTP in vitro.
Fig. 1.
Stress effects of hippocampal LTP and MWM. (A) Synaptic strength is expressed as a percentage of the average pretetanus f-EPSP over time. (Inset) A sample (average of nine successive) f-EPSP, before and after tetanus. (B) Hidden platform escape latencies during the acquisition phase. (C) Swim speed during acquisition. (D) Percent time spent swimming in four quadrant zones during the probe test. (E) Number of annulus crossing and latency to first cross the platform location during the probe test. (F) Visible platform escape latencies during the acquisition phase (15-min intertrial interval). (G) Swim distance during acquisition.
Stress Effects on Morris Water Maze (MWM).
Experimental rats were exposed to audiogenic stress terminating 30–60 min before training in the MWM, whereas control rats were not exposed to stress. In a hidden platform task (cf. ref. 42), both control (n = 12) and stress (n = 12) rats improved their performance to find the platform during the 16 training trials. The acquisition rate was comparable between the two groups, as measured by the latency to find the platform (Fig. 1B; P = 0.520, main effect of group; P = 0.978, group × trial interaction; one-way ANOVA with trial as a repeated measure) and the distance to the platform (P = 0.438, main effect of group; P = 0.992, group × trial interaction). The control and stressed rats also exhibited similar swim speeds during acquisition (Fig. 1C; P = 0.451, main effect of group; P = 0.619, group × trial interaction). On the probe test 24 h later, however, there were notable group differences on spatial memory retention. Specifically, the control animals spent more time swimming in the quadrant where the escape platform was placed during training than the stress animals (Fig. 1D; P < 0.034; one-way ANOVA with quadrant as a repeated measure). There were no reliable group differences in the other three quadrants (P = 0.448, 0.239, and 0.063). Stressed rats also displayed a significant decrease in quadrant entries as measured by annulus crossing compared with control rats (Fig. 1E; P = 0.001). There was also a trend toward control rats initially swimming to the original platform location quicker than stressed rats (P = 0.123).
Another group of stress (n = 8) and control (n = 8) rats was tested on a visible platform task (42). During training, the acquisition rate was comparable between the groups, as shown by the latency to find the platform (Fig. 1F; P = 0.289, main effect of group; P = 0.973, group × trial interaction) and the distance to the platform (Fig. 1G; P = 0.343, main effect of group; P = 0.834, group × trial interaction). During the retention test 24 h later, although not significant, there were trends of stress animals reaching the visible platform quicker than control animals (latency: P = 0.116; distance: P = 0.152). Collectively, these results indicate that stress selectively impaired the consolidation of spatial memory, without altering the sensory motor systems (abilities to navigate using local and distal cues and swim) or the motivation to escape the water.
Stress Effects on Place Cells.
Rats (n = 8) were chronically implanted with tetrode arrays in the hippocampus CA1 layer (see Materials and Methods). Place cells were recorded while the rat foraged freely for food pellets on a novel open-field platform that was located in the center of a familiar room. The recording room and extramaze cues were the same throughout all screening and recording sessions, but on each experiment day the platform differed in size, shape, texture, or orientation with respect to the surrounding room. Two recording sessions were conducted on each experiment day. During session 1, rats foraged for 15 min on the novel platform. Rats were then removed from the platform and given either control or audiogenic stress treatment. The rat was then returned to the platform in exactly the same configuration as in session 1, and place cells were again recorded for an additional 15 min (session 2). Over a period of 3–4 weeks, each rat received several administrations of the control and stress treatments in counterbalanced order, using a novel platform configuration on each experiment day.
From eight rats, a total of 55 place cells were recorded during control treatments and 49 place cells were recorded during stress treatments. A blind matching algorithm [see supporting information (SI) Text] was used to select an equal-sized subset of cells from each treatment condition (n = 36) that had nearly identical distributions of firing properties before treatment (see Table 1 for session means) so that any observed effects of the stress treatment would not be attributable to inhomogeneities in the two cell populations before treatment. All of the rats contributed similar proportions of data to the cell population for each treatment condition, so treatment effects were not attributable to inclusion of cells from different subjects in each condition.
Table 1.
Treatment means for place cell firing properties during sessions 1 and 2
| Property | Session 1 |
Session 2 |
||
|---|---|---|---|---|
| Control | Stressed | Control | Stressed | |
| Field size, cm2 | 700 ± 30 | 672 ± 444 | 649 ± 37 | 644 ± 47 |
| Peak rate, Hz | 17.6 ± 1.5 | 17.6 ± 2.2 | 14.5 ± 1.4 | 13.5 ± 1.9 |
| Spatial info, bits per s | 5.9 ± 0.7 | 5.9 ± 0.7 | 4.6 ± 0.7 | 4.1 ± 0.6 |
| Running speed, cm/s | 16.4 ± 0.7 | 15.2 ± 0.6 | 16.1 ± 0.8 | 15.3 ± 0.6 |
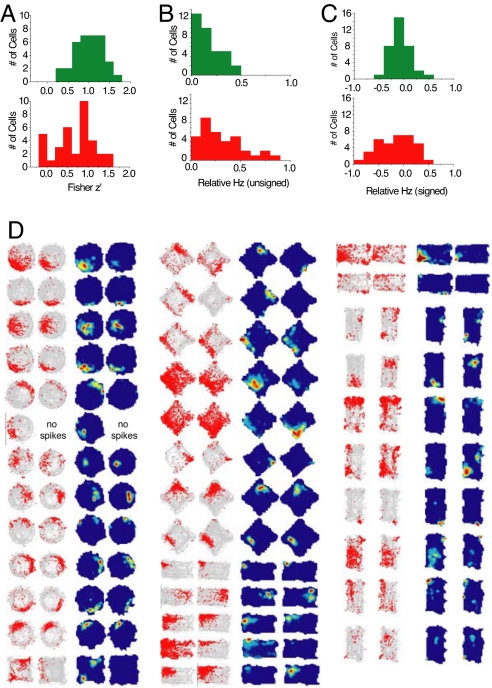
Fig. 2D shows all of the place cells (n = 36) analyzed in the stress group. In both groups, place cells rarely changed their preferred firing locations between sessions 1 and 2. A pixel-by-pixel correlation analysis was performed to measure the similarity between firing rate maps during sessions 1 and 2 on each experiment day. Overall, firing rate maps were well correlated between sessions 1 and 2 for both treatment conditions (Table 2), but the correlation across sessions was significantly reduced in the stressed versus control condition (z′stress < z′control) independent t tests; P = 0.003), suggesting that stress may have impaired the stability of spatial representations in the hippocampus (Fig. 2A). The stress and control groups showed no significant difference (independent t test; P = 0.74) in the distance between the pixel bin with the highest firing rate during session 1 versus session 2 (referred to as the “field shift”), indicating that stress did not corrupt the stability of place cell preferred firing locations. However, after stress, place cells showed an increased tendency to change their firing rate without changing their preferred firing location, a phenomenon that has been referred to as “rate remapping” (43–45). Evidence for stress-induced rate remapping was obtained by comparing changes in firing rate for the control versus stress conditions (Fig. 2 A and B and Table 2). The unsigned change in firing rate between sessions [denoted Abs(ΔHz)] was significantly larger for the stress than the control condition [Abs(ΔHz)stress > Abs(ΔHz)control, independent t test; P = 0.004], suggesting that place cell firing rates were less stable across sessions when rats were stressed. The signed firing rate change between sessions (denoted ΔHz) showed a nonsignificant trend toward lower values for the stress than the control condition (ΔHzstress < ΔHzcontrol, independent t tests; P = 0.12), suggesting that firing rate decreases were slightly more common than increases after stress treatment. As explained in Discussion, these results suggest that stress may disrupt spatial memory in rodents by selectively impairing the stability of place cell firing rates.
Fig. 2.
Stress effects on hippocampal place cells. (A) Distributions (frequency histograms) of pixel by pixel cross-correlations (z transformed) between before and after stress place maps of individual place cells (green = control; red = stress). (B) Distributions of unsigned relative firing rates of before stress and after stress conditions. (C) Distributions of signed relative firing rates of before stress and after stress conditions. (D) Occupancy (or visit) maps and place maps. The left two in each group represent the occupancy maps of before stress and after stress sessions, and the right two show the place maps corresponding to the left two occupancy maps.
Table 2.
Treatment means for statistical comparisons between sessions 1 and 2
| Property | Control | Stressed |
|---|---|---|
| Spatial correlation, z′ | 0.98 ± 0.06 | 0.69 ± 0.08 |
| Unsigned relative firing rate | 0.18 ± 0.02 (1.51-fold) | 0.31 ± 0.04 (2.42-fold) |
| Unsigned relative spatial information | 0.24 ± 0.02 (1.73-fold) | 0.33 ± 0.04 (3.00-fold) |
| Unsigned relative field size | 0.10 ± 0.02 (1.25-fold) | 0.12 ± 0.02 (1.33-fold) |
| Unsigned relative running speed | 0.06 ± 0.01 (1.15-fold) | 0.09 ± 0.03 (1.26-fold) |
| Signed relative firing rate | −0.06 ± 0.04 | −0.17 ± 0.06 |
| Signed relative spatial information | −0.12 ± 0.04 | −0.23 ± 0.06 |
| Signed relative field size | −0.03 ± 0.02 | −0.02 ± 0.03 |
| Signed relative running speed | −0.01 ± 0.02 | +0.04 ± 0.04 |
Discussion
Here, we have shown that audiogenic stress impairs hippocampal LTP and spatial memory. Specifically, slices from rats exposed to stress exhibited impaired LTP in area CA1 compared with slices from control rats. Stress also impaired spatial memory in the MWM, without altering the sensory motor systems (swim speed and performance on a visible platform task) or the motivation to escape the water. Although stress impaired the retention of spatial memory, during the acquisition phase stressed rats exhibited rates of improvement in locating the hidden platform similar to those of control rats, suggesting that stress effects on LTP are correlated with retention (and not acquisition) of spatial memory. A similar finding was originally reported by Morris et al. (46), where rats infused with the NMDAR antagonist (AP5) were CA1 LTP deficient but exhibited mild (but not reliable) acquisition deficit in the MWM. They attributed this spared learning to the animal's use of “nonspecific instrumental learning” masking impairments in true place learning. It is possible then that stressed rats used a similar nonspecific strategy during acquisition. Overall, the present findings are in agreement with prior studies showing similar LTP and spatial memory impairments using different stress treatments (14–17, 20–28). However, stress effects on hippocampal place cells have not been previously investigated, and this question is important for understanding the relationship between stress and memory. Here, we have shown that the same stress that impairs hippocampal LTP and spatial memory also disrupts place cells by impairing the stability of their firing rates (but not preferred firing locations) on a novel platform in a familiar room.
Studies have shown that when rats move from one familiar spatial environment to another, place cells typically rearrange their preferred firing locations to form a new population code that represents the new environment, a phenomenon referred to as global remapping (33, 43, 47). By contrast, when recognizable cues within a single familiar environment are altered, place cells frequently respond by rearranging their firing rate distribution without changing their preferred firing locations, a phenomenon referred to as rate remapping (43–45, 48). Here, we recorded place cells in a familiar room in which cues were altered by changing the shape of the recording platform on different days, a procedure that typically induces rate mapping but not global remapping (43, 48). In both stressed and control rats, the distribution of place cell firing locations was stable across two repeated visits to the novel platform shape on the same day, in accordance with prior findings showing that changing the platform shape rarely induces global remapping (43, 48). However, we also found that the distribution of place cell firing rates was less stable across the two platform visits in stressed than control rats. One possible explanation for this finding is that unlike control rats, stressed rats failed to store a stable rate map for representing the novel platform shape after the first visit, so that the place cells underwent spontaneous rate remapping between the first and second visits. This impaired storage of stable rate maps in stressed rats might have resulted from impaired CA1 LTP. Supporting this interpretation, a recent study suggests that LTP within the hippocampus may be essential for storing stable rate maps that encode different platform shapes within a familiar room (48). More specifically, this prior study showed that CA3 place cells failed to form distinct rate maps of two different platforms in tissue-specific knockout mice exhibiting impaired DG (but not CA1) LTP. By contrast, our study showed rate instability of CA1 place cells in conjunction with impaired CA1 LTP after stress. Hence, whereas prior findings indicate that DG LTP may be critical for forming stable rate maps in CA3, our present findings indicate that CA1 LTP may be critical for forming stable rate maps in CA1. These conclusions are not incompatible with one another, but it should be noted that stress impairs LTP in DG and in CA1 (26), so although we did not test LTP in DG, it is possible that stress-induced impairments of dentate LTP (in conjunction with observed CA1 LTP deficits) may have contributed to the rate map instability after stress in our experiments.
Leutgeb et al. (43–45) proposed that the formation of stable firing rate maps by hippocampal place cells may provide a mechanism for encoding episodic memories about events that have previously occurred within a given spatial context. According to this view, the pattern of preferred firing locations of place cells encodes the identity of the rat's spatial environment (“spatial memory”), whereas the pattern of place cell firing rates encodes information about cues or events occurring within that environment (“episodic memory”). If so, then our present findings suggest that one mechanism by which stress might disrupt memory processing is by impairing the ability of the hippocampus to store stable place cell firing rate maps, which could in turn lead to deficits in episodic memory coding. Such episodic memory deficits could account for impairments in behavioral tasks, including the MWM. We found that probe trial performance in the hidden (but not visible) platform MWM task was impaired by the same audiogenic stress that disrupted hippocampal LTP and firing rate stability of place cells. The MWM task (42) probably depends on both spatial and episodic memory functions of the hippocampus, because to find the hidden platform rats must construct a “cognitive map” that stores a spatial memory of the maze environment (this may require global remapping of place cells during early exposure to the maze), and they must also store episodic memories of where the platform was located on each learning trial (this may require rate remapping of place cells to “mark” the location of the platform within the cognitive map over the course of learning). Our present findings suggest that stress can impair the formation of stable place cell firing rate maps, which may impair water maze performance by disrupting the rat's ability to form a stable rate map that marks the location of the hidden platform. This conjecture needs further testing by using spaced training across days (to minimize confounds of consolidation and incremental learning) and variable platform locations (to vary the episodic demand) to disambiguate episodic vs. spatial aspects of the MWM task affected by stress.
Another question that remains unanswered by our present findings is whether stress can disrupt not only the stability of place cell firing rates (as shown here), but also disrupt the stability of place cell firing locations (which might impair water maze performance by disrupting the rat's ability to construct a cognitive map of the maze environment). In the present study, we found that stress produced spontaneous rate remapping but not global remapping of place cells. However, the experimental procedure we used (recording place cells on a novel platform in a familiar surrounding room) normally induces rate remapping and not global remapping (43, 48). To further test whether stress can also impair the stability of place cell firing locations and thereby induce global remapping of place cells, it would be necessary to expose rats to stress when they are first introduced into a completely novel and unfamiliar environment, because this is the time when the preferred firing locations of place cells are thought to be formed by hippocampal plasticity. If stress-induced disruption of hippocampal LTP is responsible for impaired formation of stable rate maps when the rat first encounters a novel platform in a familiar room (as we have shown), then a similar disruption of LTP might impair formation of stable preferred firing locations if it occurs at the time when the rat encounters a new room for the first time.
Stress-induced impairment in LTP has been hypothesized as a biological cause for memory impediments associated with stress (3–6). How might stress effects on LTP be related to stress effects on place cell firing rates and episodic memory? Hippocampal plasticity might be required to “stamp in” a specific firing rate pattern that stores a specific episodic memory representation within the place cell population. Therefore, because stress impairs hippocampus plasticity (20–28), it is possible that stress induces rate remapping by interfering with synaptic modifications that are needed to form stable rate maps within the hippocampus.
In summary, we found that audiogenic stress alters three related levels of hippocampal processing: LTP, spatial memory, and place cell activity. Consistent with effects on LTP and spatial memory, stress impaired the stability of place cell firing rates with only mildly affecting their preferred firing locations. These findings contribute to a growing body of evidence that the distribution of place cell firing rates in the hippocampus encodes behaviorally relevant information (43–45, 48) and provide evidence that memory performance is impaired by manipulations (such as stress) that disrupt the stability of this firing rate distribution. The stress-induced rate remapping of place cells we have observed in this study might therefore help to explain how stress impairs memory in hippocampal-dependent tasks.
Materials and Methods
Animals and Stress Paradigm.
In all experiments, male Charles River Long-Evans rats (initially weighing 270–300 g) were placed on a standard food deprivation schedule to gradually reach and maintain 80–85% of their original weight. The audiogenic stress procedure involved placing the animals in a well lit novel chamber for 2 h and presenting 1-s bursts of 100 dB of white noise at 30–90 s apart. This stress is commonly used in human, nonhuman primate, and rodent studies (1) and reliably activates the hypothalamus–pituitary–adrenal axis (39, 40). All experiments were conducted in compliance with the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services). The slice and water maze experiments were completed at Yale University, New Haven, CT (J.J.K.'s former laboratory), and the place cell recordings were performed at the University of California, Los Angeles (H.T.B.'s laboratory).
Slice Recording.
Promptly after stress, hippocampal slices were prepared as described (18, 25, 41). In brief, transverse slices (400 μm) were maintained in an interface recording chamber continuously perfused (≈2 ml/min) with 95% O2 and 5% CO2 saturated artificial cerebrospinal fluid (124 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1 mM MgSO2, 26 mM NaHCO3, 3 mM CaCl2, and 10 mM glucose) at 32°C ± 0.5°C. After ≥1 h of incubation, the Schaffer collateral/commissural fibers were stimulated by concentric bipolar electrodes (25 μm inner contact diameter) that delivered 100-μs pulses. Glass electrodes filled with 2 M NaCl were placed in CA1 stratum radiatum to record f-EPSPs. A standard I/O curve was generated, and test stimulus intensity was adjusted to produce a response that was 50% of the maximum evoked responses. Baseline synaptic transmission was monitored for 20 min before delivering a tetanus (five trains of 100 Hz, each lasting 200 ms at an intertrain interval of 10 s). The f-EPSPs, amplified in the band of 0.1–5,000 Hz, were monitored every 20 s, up to 1 h after the tetanus. During tetanus, f-EPSPs evoked by the first pulse in each of the five trains were recorded to monitor the development of potentiation. Data were collected and analyzed with programs written in Axobasic/Quickbasic (Axon Instruments, Union City, CA). The negative slope of f-EPSPs was used in statistical analyses, and the change in f-EPSPs after tetanus was averaged per minute across two slices for each rat. The LTP was measured between 40 and 60 min after tetanus and compared with the 20-min baseline measure.
Water Maze Task.
The procedures for the hidden platform were adapted from published protocol (17, 42). After stress, rats returned to their home cages for 30–60 min before undergoing 16 massed training trials (15 min intertrial interval) to find a fixed submerged platform (10 cm diameter) and escape from a circular water maze (2.0 m diameter, 0.7 m high) filled with water (∼23°C) made opaque by the addition of profax resin pellets. The starting point was randomly distributed across the four quadrants of the pool (four starting points per quadrant). If escape did not occur within 60 s, the animal was manually guided to the platform. Upon finding the platform, the animal remained on the platform for 60 s, and then placed in a holding cage for 15 min before the next trial. The next day, a retention test (a 60-s probe trial) was given in which the platform was removed from the pool. Animals' swimming pattern, the number of platform crossings, and the amount of time spent swimming in one of the four quadrants were monitored automatically by using a computerized tracking system (HVS Image, Hampton, U.K.).
In the visible platform task, animals underwent eight training trials (15-min intertrial interval) to find a submerged platform marked with a visually noticeable flag stimulus. The platform was moved to a different location (90° shift clockwise) from trial to trial, and the starting point was randomly distributed across the four quadrants of the pool. The next day, rats were given a single retention test to find the visible platform.
Place Cell Recording and Analyses.
Before surgery, rats were reduced to 85% of their ad lib weight through limited daily feeding, so that they would be motivated to forage for food pellets during the experiment. Under isoflourane anesthesia, rats were implanted with a microdrive array loaded with six tetrodes at coordinates 3.0 mm posterior, 2.0 mm lateral, and 1.7 mm ventral to bregma. Tetrodes were made by rotating four strands of formvar-insulated nichrome wires (Kanthal, Palm Coast, FL) and gently heated to fuse the insulation. The electrode tips were cut and gold-plated to reduce impedance to 0.1–0.4 MΩ measured at 1 kHz. After recovery, tetrodes were gradually advanced (≤150 μm per day) until complex spike cells were encountered in the CA1 layer, which was identified on the basis of electroencephalogram signals and single-unit spike patterns (49). Unit signals were amplified (×10,000), filtered (600 Hz to 6 kHz), and digitized (32 kHz) by using the Cheetah data acquisition system (Neuralynx, Tuscon, AZ). The rat's head position was sampled at 30 Hz by tracking an array of light-emitting diodes mounted on the headstage.
Single units were isolated by using a combination of automated and manual cluster cutting (Neuralynx SpikeSort3D software). Place cells met several criteria for inclusion in the study: peak firing rate >3 Hz, spatial information >1.0 bits per s, stable well discriminated complex spike waveforms, and a refractory period of at least 1 ms. Recording locations were verified histologically by small marking lesions.
Three different recording platforms were used in the study: a circular platform made of black plastic (90 cm diameter), a square platform painted with beige latex (1 m2), and a rectangular platform made of gray metal (92 × 30 cm). In some sessions, the square platform was rotated by 45° and the rectangular platform was rotated by 90° relative to the surrounding environment to obtain additional novel platform configurations, so that there were a total of five different platform configurations: circle, square 0°, square 45°, rectangle 0°, and rectangle 90°. The platform was centered within a small square room (2 m2). Two adjacent walls were painted blue (one of these walls displaying a black-and-white checkerboard pattern contact paper). The wall opposite the checkerboard was a black felt curtain through which the rat always entered and left the environment. The fourth wall was made of brown particle board. This arrangement of distal cues remained identical throughout all of the experiments. Each rat was assigned a different starting platform (one of the five platform configurations) where it was screened daily until place cells were isolated in CA1. On experiment days, the starting platform was replaced with a novel platform configuration for recording sessions 1 and 2.
Firing rate maps consisted of square pixels measuring 3.25 cm on each side. Undersampled pixel bins (those visited for <1 s during either the before or after stress session) were excluded. Firing rate in each bin was the number of spikes fired divided by the time spent in that bin. Rate maps were smoothed by a single iteration of convolution with a Gaussian kernel spanning a 3 × 3-pixel region. Peak in-field firing rate was the rate of the highest pixel bin in the smoothed rate map. Field size was the summed extent (in cm2) of all contiguous regions of pixel bins whose firing rate exceeds the mean rate by at least one standard error. Spatial information content in bits per second was computed by standard methods (50). Contour plots were generated by using MATLAB software (Mathworks, Natick, MA).
A pixel-by-pixel correlation analysis was performed to measure the similarity between firing rate maps during sessions 1 and 2 for each place cell. A Fisher z′ transformation was performed on the resulting r values to compare correlation values across treatments by using parametric statistics (Fig. 2A and Table 1). Firing rate changes between sessions 1 and 2 were compared by computing both signed (ΔHz) and unsigned [Abs(ΔHz)] firing rate change values for each cell (see ref. 43). The signed firing rate change was defined as (R2 − R1)/(R2 + R1), where R1 and R2 were the peak in-field firing rates of the cell during sessions 1 and 2, respectively. The unsigned firing rate change was computed in a similar manner as Abs(R2 − R1)/(R1 + R2). The x-fold change in firing rate between sessions (Table 2) was computed as max(R1,R2)/min(R1,R2).
Supplementary Material
Acknowledgments
This work was supported by Whitehall Foundation Grant 2004-12–28 (to H.T.B.), National Institutes of Health Grant R01MH64457 (to J.J.K.), and a Royalty Research Fellowship from the University of Washington (to J.J.K.).
Abbreviations
- LTP
long-term potentiation
- CA1
Cornu Ammonis field 1
- DG
dentate gyrus
- f-EPSP
field excitatory postsynaptic potential
- MWM
Morris water maze.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708644104/DC1.
References
- 1.Selye H. Am Sci. 1973;61:692–699. [PubMed] [Google Scholar]
- 2.Maier SF, Seligman MEP. J Exp Psychol Gen. 1976;105:3–45. [Google Scholar]
- 3.McEwen BS, Sapolsky RM. Curr Opin Neurobiol. 1995;5:205–215. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 4.Kim JJ, Diamond DM. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 5.Joels M, Pu Z, Wiegert O, Oitzl MS, Druger HJ. Trends Cognit Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Shors TJ. Annu Rev Psychol. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scoville WB, Milner B. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. New York: Oxford Univ Press; 1978. [Google Scholar]
- 9.Eichenbaum H. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 10.Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, Alderson AL. Arch Gen Psychiatry. 1994;56:527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- 11.de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Nat Neurosci. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- 12.Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, Innis RB, McCarthy G, Charney DS. Am J Psychiatry. 1993;150:1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- 13.Utto M, Vasterling JJ, Brailey K, Sutker PB. J Psychopathol Behav Assess. 1993;15:43–52. [Google Scholar]
- 14.Luine VN, Spencer RL, McEwen BS. Brain Res. 1993;616:65–70. doi: 10.1016/0006-8993(93)90193-q. [DOI] [PubMed] [Google Scholar]
- 15.Diamond DM, Rose GM. Ann NY Acad Sci. 1994;746:411–414. doi: 10.1111/j.1749-6632.1994.tb39271.x. [DOI] [PubMed] [Google Scholar]
- 16.de Quervain DJ-F, Roozendaal B, McGaugh JL. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 17.Kim JJ, Koo JW, Lee HJ, Han JS. J Neurosci. 2005;25:1532–1539. doi: 10.1523/JNEUROSCI.4623-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bliss TVP, Lomo T. J Physiol (London) 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin SJ, Grimwood PD, Morris RG. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 20.Foy MR, Stanton ME, Levine S, Thompson RF. Behav Neural Biol. 1987;48:138–149. doi: 10.1016/s0163-1047(87)90664-9. [DOI] [PubMed] [Google Scholar]
- 21.Shors TJ, Seib TB, Levine S, Thompson RF. Science. 1989;244:224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- 22.Diamond DM, Park CR. Ann NY Acad Sci. 2000;911:453–455. doi: 10.1111/j.1749-6632.2000.tb06743.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim JJ, Foy MR, Thompson RF. Proc Natl Acad Sci USA. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akirav I, Richter-Levin G. J Neurosci. 1999;19:10530–10535. doi: 10.1523/JNEUROSCI.19-23-10530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu L, Anwyl R, Rowan MJ. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- 26.Shors TJ, Dryver E. Brain Res. 1994;666:232–238. doi: 10.1016/0006-8993(94)90777-3. [DOI] [PubMed] [Google Scholar]
- 27.Shors TJ, Gallegos RA, Breindl A. Synapse. 1997;26:209–217. doi: 10.1002/(SICI)1098-2396(199707)26:3<209::AID-SYN2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 28.Shors TJ, Levine S, Thompson RF. Neuroendocrinology. 1990;51:70–75. doi: 10.1159/000125318. [DOI] [PubMed] [Google Scholar]
- 29.O'Keefe J, Dostrovsky J. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 30.Jung MW, McNaughton BL. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- 31.Muller RU, Kubie JL. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson LT, Best PJ. Brain Res. 1990;509:2299–2308. doi: 10.1016/0006-8993(90)90555-p. [DOI] [PubMed] [Google Scholar]
- 33.Wilson MA, McNaughton BL. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 34.McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA. Cell. 1996;87:1339–1349. doi: 10.1016/s0092-8674(00)81828-0. [DOI] [PubMed] [Google Scholar]
- 35.Cho YH, Giese KP, Tanila H, Silva AJ, Eichenbaum H. Science. 1998;279:867–869. doi: 10.1126/science.279.5352.867. [DOI] [PubMed] [Google Scholar]
- 36.Kentros C, Hargreaves E, Hawking RD, Kandel ER, Shapiro M, Muller RV. Science. 1998;280:2121–2126. doi: 10.1126/science.280.5372.2121. [DOI] [PubMed] [Google Scholar]
- 37.Rotenberg A, Mayford M, Hawkins RD, Kandel ER, Muller RU. Cell. 1996;87:1351–1361. doi: 10.1016/s0092-8674(00)81829-2. [DOI] [PubMed] [Google Scholar]
- 38.Dragoi G, Harris KD, Buzsaki G. Neuron. 2003;39:843–853. doi: 10.1016/s0896-6273(03)00465-3. [DOI] [PubMed] [Google Scholar]
- 39.Campeau S, Akil H, Watson SJ. J Neurosci. 1997;17:5979–5992. doi: 10.1523/JNEUROSCI.17-15-05979.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palkovits M, Dobolyi A, Helfferich F, Usdin TB. Ann NY Acad Sci. 2004;1018:16–24. doi: 10.1196/annals.1296.002. [DOI] [PubMed] [Google Scholar]
- 41.Teyler TJ. Brain Res Bull. 1980;5:391–403. doi: 10.1016/s0361-9230(80)80009-8. [DOI] [PubMed] [Google Scholar]
- 42.Morris RGM, Garrud P, Rawlins JNP, O'Keefe J. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 43.Leutgeb S, Leutgeb JK, Treves A, Moser M-B, Moser EI. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- 44.Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser M-B. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- 45.Leutgeb S, Leutgeb JK, Moser M-B, Moser EI. Hippocampus. 2006;16:765–774. doi: 10.1002/hipo.20201. [DOI] [PubMed] [Google Scholar]
- 46.Morris RGM, Anderson E, Lynch GS, Baudry M. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 47.Bostock E, Muller RU, Kubie JL. Hippocampus. 1991;1:193–205. doi: 10.1002/hipo.450010207. [DOI] [PubMed] [Google Scholar]
- 48.McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 49.Buzsaki G. Brain Res. 1986;398:242–248. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- 50.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Hippocampus. 1996;6:271–280. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.