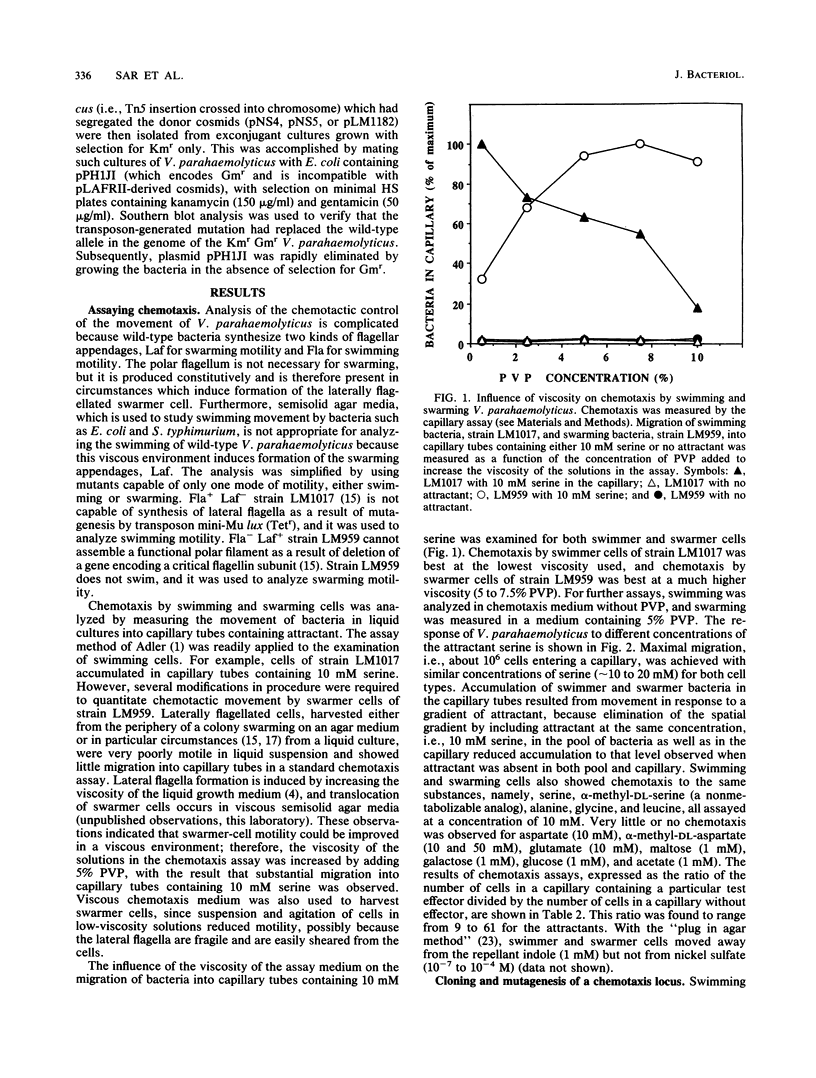
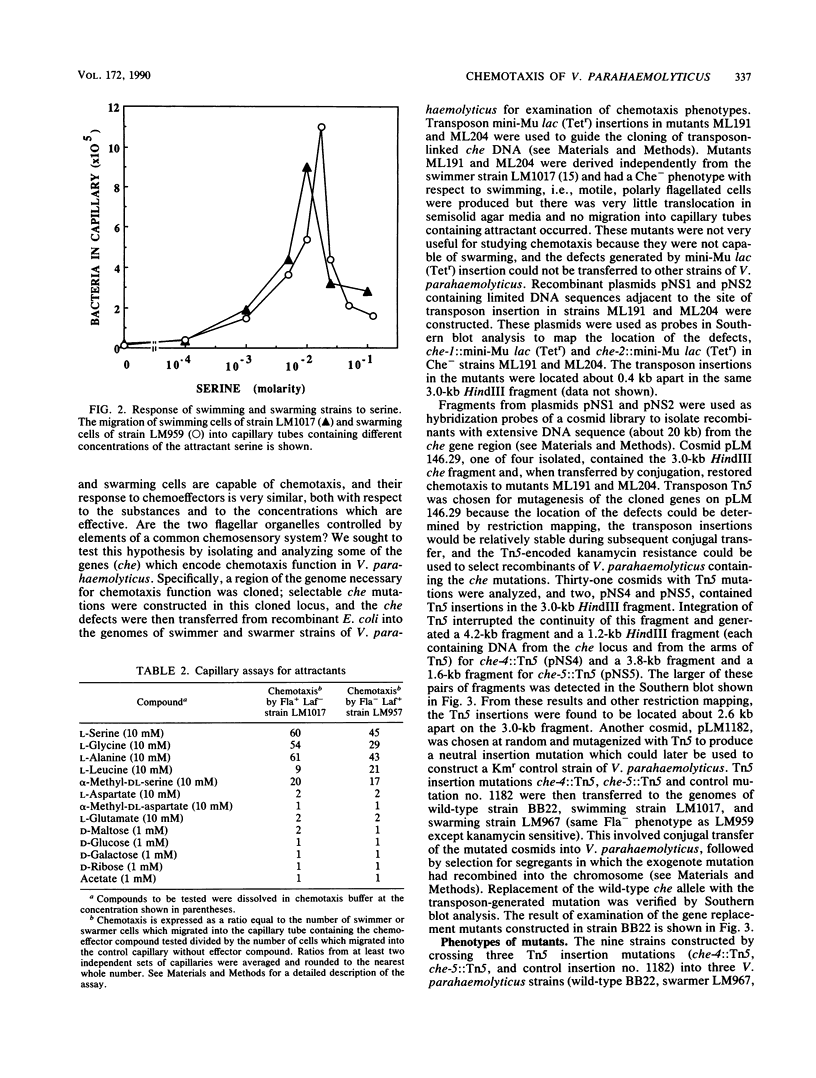
Abstract
Vibrio parahaemolyticus synthesizes two distinct flagellar organelles, the polar flagellum (Fla), which propels the bacterium in a liquid environment (swimming), and the lateral flagella (Laf), which are responsible for movement over surfaces (swarming). Chemotactic control of each of these flagellar systems was evaluated separately by analyzing the behavioral responses of strains defective in either motility system, i.e., Fla+ Laf- (swimming only) or Fla- Laf+ (swarming only) mutants. Capillary assays, modified by using viscous solutions to measure swarming motility, were used to quantitate chemotaxis by the Fla+ Laf- or Fla- Laf+ mutants. The behavior of the mutants was very similar with respect to the attractant compounds and the concentrations which elicited responses. The effect of chemotaxis gene defects on the operation of the two flagellar systems was also examined. A locus previously shown to encode functions required for chemotactic control of the polar flagellum was cloned and mutated by transposon Tn5 insertion in Escherichia coli, and the defects in this locus, che-4 and che-5, were then transferred to the Fla+ Laf- or Fla- Laf+ strains of V. parahaemolyticus. Introduction of the che mutations into these strains prevented chemotaxis into capillary tubes and greatly diminished movement of bacteria over the surface of agar media or through semisolid media. We conclude that the two flagellar organelles, which consist of independent motor-propeller structures, are directed by a common chemosensory control system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Belas R., Mileham A., Simon M., Silverman M. Transposon mutagenesis of marine Vibrio spp. J Bacteriol. 1984 Jun;158(3):890–896. doi: 10.1128/jb.158.3.890-896.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R., Simon M., Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986 Jul;167(1):210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Borkovich K. A., Kaplan N., Hess J. F., Simon M. I. Transmembrane signal transduction in bacterial chemotaxis involves ligand-dependent activation of phosphate group transfer. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1208–1212. doi: 10.1073/pnas.86.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter L. L., Silverman M. Phosphate regulation of gene expression in Vibrio parahaemolyticus. J Bacteriol. 1987 Aug;169(8):3441–3449. doi: 10.1128/jb.169.8.3441-3449.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter L., Hilmen M., Silverman M. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988 Jul 29;54(3):345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- McCarter L., Silverman M. Iron regulation of swarmer cell differentiation of Vibrio parahaemolyticus. J Bacteriol. 1989 Feb;171(2):731–736. doi: 10.1128/jb.171.2.731-736.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Ausubel F. M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- Shinoda S., Honda T., Takeda Y., Miwatani T. Antigenic difference between polar montrichous and peritrichous flagella of Vibrio parahaemolyticus. J Bacteriol. 1974 Nov;120(2):923–928. doi: 10.1128/jb.120.2.923-928.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda S., Okamoto K. Formation and function of Vibrio parahaemolyticus lateral flagella. J Bacteriol. 1977 Mar;129(3):1266–1271. doi: 10.1128/jb.129.3.1266-1271.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Chemotaxis in Escherichia coli: methylation of che gene products. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3317–3321. doi: 10.1073/pnas.74.8.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M. S., Goy M. F., Adler J. Sensory transduction in Escherichia coli: two complementary pathways of information processing that involve methylated proteins. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3312–3316. doi: 10.1073/pnas.74.8.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso W. W., Adler J. Negative chemotaxis in Escherichia coli. J Bacteriol. 1974 May;118(2):560–576. doi: 10.1128/jb.118.2.560-576.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzer S. The mechanism of swarming of Vibrio alginolyticus. Arch Microbiol. 1975 Jun 20;104(1):67–71. doi: 10.1007/BF00447301. [DOI] [PubMed] [Google Scholar]
- Williams F. D., Anderson D. M., Hoffman P. S., Schwarzhoff R. H., Leonard S. Evidence against the involvement of chemotaxis in swarming of Proteus mirabilis. J Bacteriol. 1976 Jul;127(1):237–248. doi: 10.1128/jb.127.1.237-248.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]




