Abstract
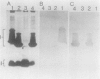
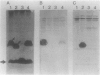
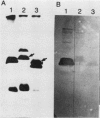
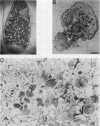
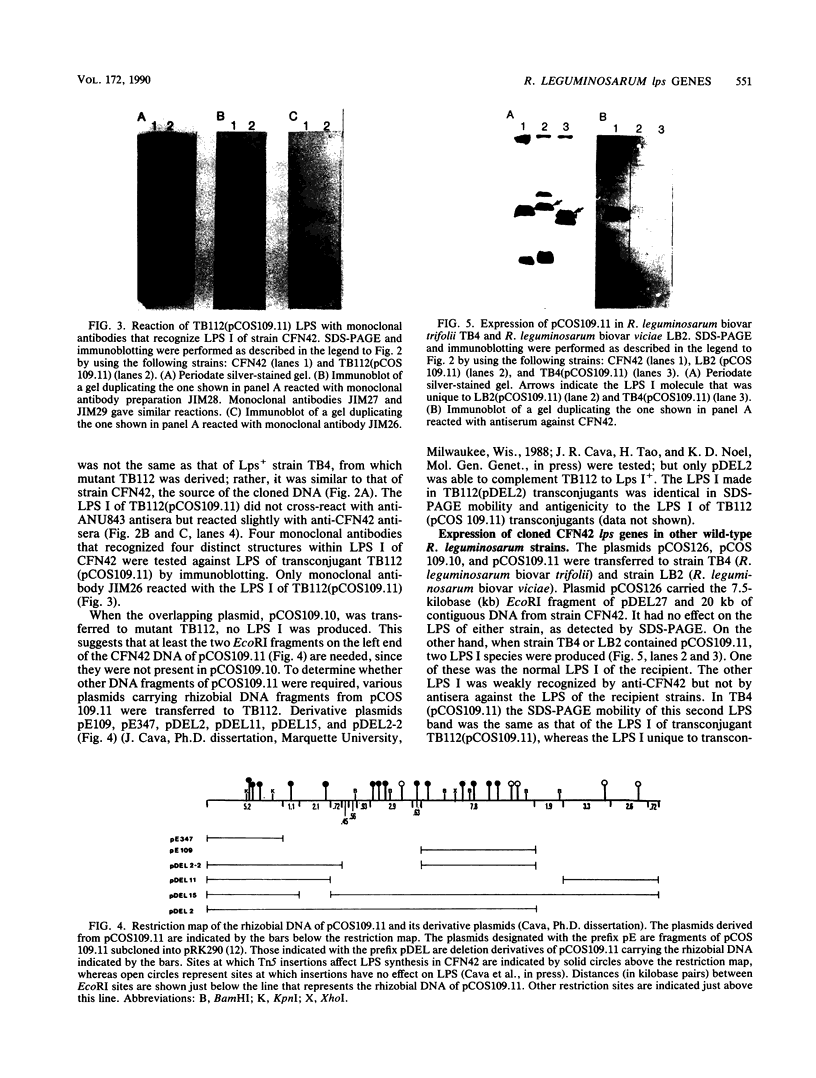
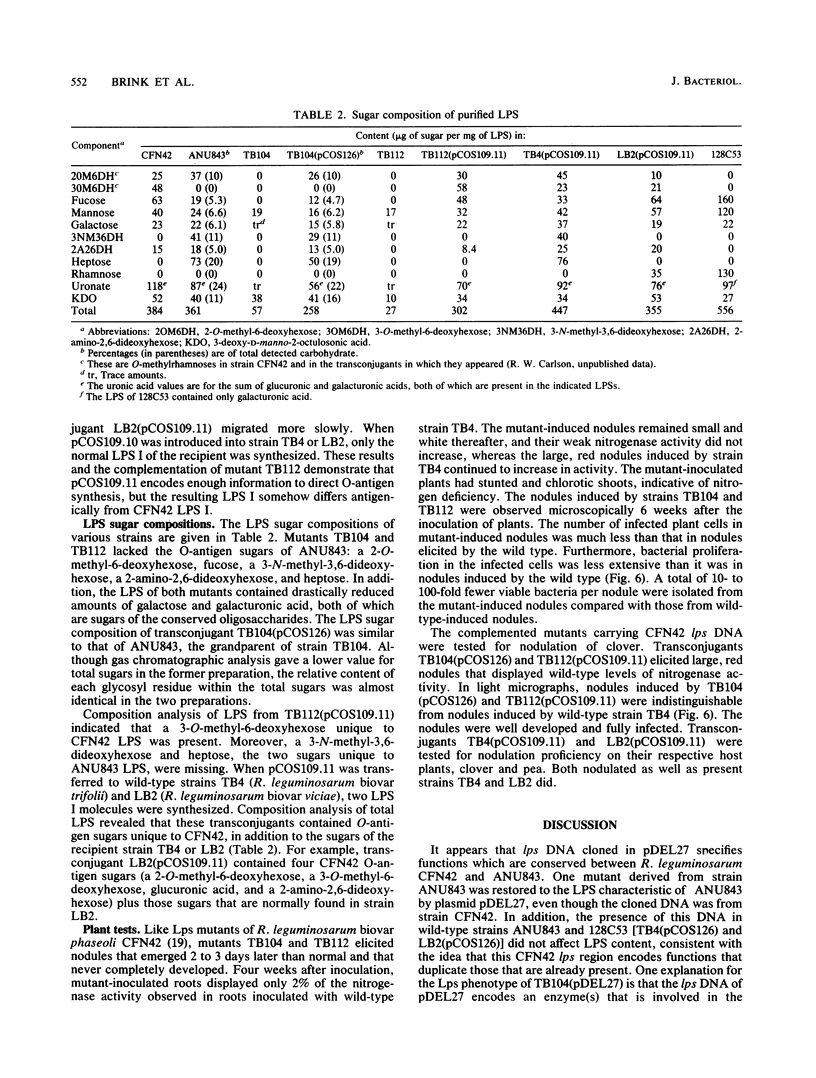
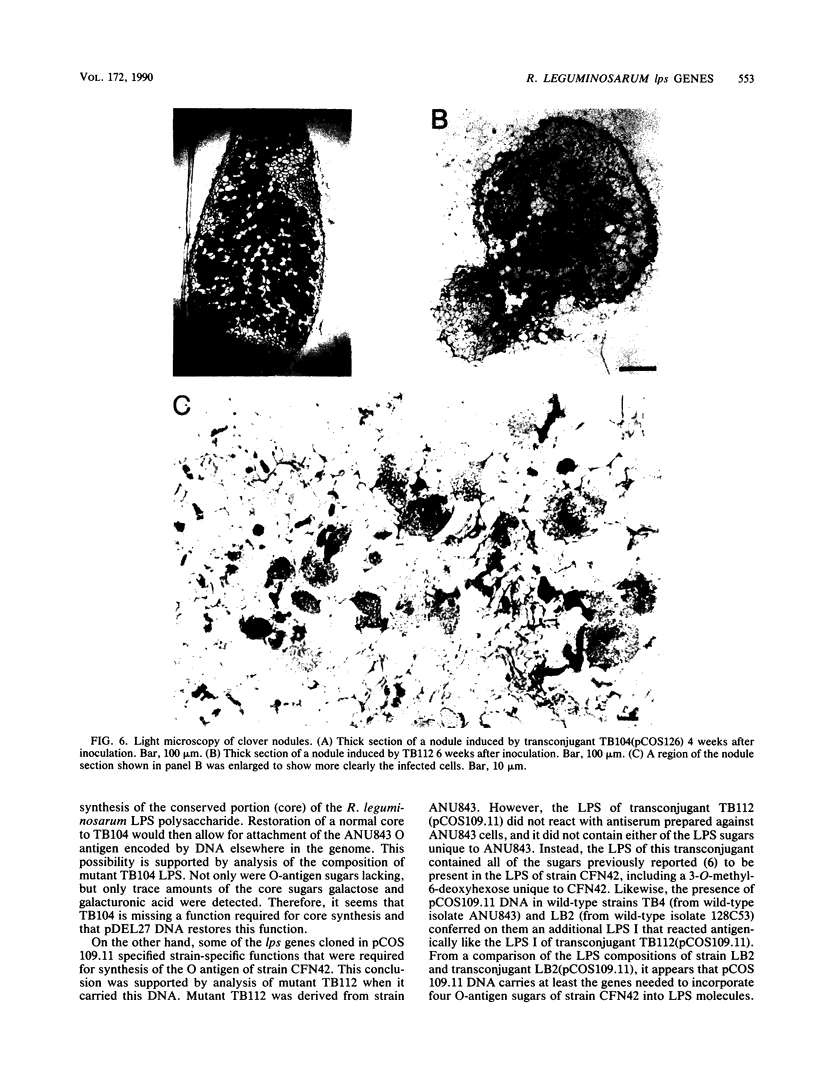
Two mutant derivatives of Rhizobium leguminosarum ANU843 defective in lipopolysaccharide (LPS) were isolated. The LPS of both mutants lacked O antigen and some sugar residues of the LPS core oligosaccharides. Genetic regions previously cloned from another Rhizobium leguminosarum wild-type isolate, strain CFN42, were used to complement these mutants. One mutant was complemented to give LPS that was apparently identical to the LPS of strain ANU843 in antigenicity, electrophoretic mobility, and sugar composition. The other mutant was complemented by a second CFN42 lps genetic region. In this case the resulting LPS contained O-antigen sugars characteristic of donor strain CFN42 and reacted weakly with antiserum against CFN42 cells, but did not react detectably with antiserum against ANU843 cells. Therefore, one of the CFN42 lps genetic regions specifies a function that is conserved between the two R. leguminosarum wild-type isolates, whereas the other region, at least in part, specifies a strain-specific LPS structure. Transfer of these two genetic regions into wild-type strains derived from R. leguminosarum ANU843 and 128C53 gave results consistent with this conclusion. The mutants derived from strain ANU843 elicited incompletely developed clover nodules that exhibited low bacterial populations and very low nitrogenase activity. Both mutants elicited normally developed, nitrogen-fixing clover nodules when they carried CFN42 lps DNA that permitted synthesis of O-antigen-containing LPS, regardless of whether the O antigen was the one originally made by strain ANU843.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum E. R., Thompson D. V., Idler K., Chartrain N. Rhizobium japonicum USDA 191 has two nodD genes that differ in primary structure and function. J Bacteriol. 1988 Jan;170(1):12–20. doi: 10.1128/jb.170.1.12-20.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Carlson R. W. Heterogeneity of Rhizobium lipopolysaccharides. J Bacteriol. 1984 Jun;158(3):1012–1017. doi: 10.1128/jb.158.3.1012-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Hollingsworth R. L., Dazzo F. B. A core oligosaccharide component from the lipopolysaccharide of Rhizobium trifolii ANU843. Carbohydr Res. 1988 May 1;176(1):127–135. doi: 10.1016/0008-6215(88)84064-3. [DOI] [PubMed] [Google Scholar]
- Carlson R. W., Kalembasa S., Turowski D., Pachori P., Noel K. D. Characterization of the lipopolysaccharide from a Rhizobium phaseoli mutant that is defective in infection thread development. J Bacteriol. 1987 Nov;169(11):4923–4928. doi: 10.1128/jb.169.11.4923-4928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Shatters R., Duh J. L., Turnbull E., Hanley B., Rolfe B. G., Djordjevic M. A. The Isolation and Partial Characterization of the Lipopolysaccharides from Several Rhizobium trifolii Mutants Affected in Root Hair Infection. Plant Physiol. 1987 Jun;84(2):421–427. doi: 10.1104/pp.84.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cava J. R., Elias P. M., Turowski D. A., Noel K. D. Rhizobium leguminosarum CFN42 genetic regions encoding lipopolysaccharide structures essential for complete nodule development on bean plants. J Bacteriol. 1989 Jan;171(1):8–15. doi: 10.1128/jb.171.1.8-15.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clover R. H., Kieber J., Signer E. R. Lipopolysaccharide mutants of Rhizobium meliloti are not defective in symbiosis. J Bacteriol. 1989 Jul;171(7):3961–3967. doi: 10.1128/jb.171.7.3961-3967.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold R., Noel K. D. Rhizobium leguminosarum exopolysaccharide mutants: biochemical and genetic analyses and symbiotic behavior on three hosts. J Bacteriol. 1989 Sep;171(9):4821–4830. doi: 10.1128/jb.171.9.4821-4830.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth R. I., Carlson R. W., Garcia F., Gage D. A. A new core tetrasaccharide component from the lipopolysaccharide of Rhizobium trifolii ANU 843. J Biol Chem. 1989 Jun 5;264(16):9294–9299. [PubMed] [Google Scholar]
- Noel K. D., Sanchez A., Fernandez L., Leemans J., Cevallos M. A. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J Bacteriol. 1984 Apr;158(1):148–155. doi: 10.1128/jb.158.1.148-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Vandenbosch K. A., Kulpaca B. Mutations in Rhizobium phaseoli that lead to arrested development of infection threads. J Bacteriol. 1986 Dec;168(3):1392–1401. doi: 10.1128/jb.168.3.1392-1401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Gerhold D., Stacey G. Cell surface polysaccharides from Bradyrhizobium japonicum and a nonnodulating mutant. J Bacteriol. 1987 Jan;169(1):137–141. doi: 10.1128/jb.169.1.137-141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbosch K. A., Noel K. D., Kaneko Y., Newcomb E. H. Nodule initiation elicited by noninfective mutants of Rhizobium phaseoli. J Bacteriol. 1985 Jun;162(3):950–959. doi: 10.1128/jb.162.3.950-959.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maagd R. A., Rao A. S., Mulders I. H., Goosen-de Roo L., van Loosdrecht M. C., Wijffelman C. A., Lugtenberg B. J. Isolation and characterization of mutants of Rhizobium leguminosarum bv. viciae 248 with altered lipopolysaccharides: possible role of surface charge or hydrophobicity in bacterial release from the infection thread. J Bacteriol. 1989 Feb;171(2):1143–1150. doi: 10.1128/jb.171.2.1143-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]