Abstract
The PDC1 gene coding for a pyruvate decarboxylase (PDC; EC 4.1.1.1) was deleted from the Saccharomyces cerevisiae genome. The resulting pdc1(0) mutants were able to grow on glucose and still contained 60 to 70% of the wild-type PDC activity. Two DNA fragments with sequences homologous to that of the PDC1 gene were cloned from the yeast genome. One of the cloned genes (PDC5) was expressed at high rates predominantly in pdc1(0) strains and probably encodes the remaining PDC activity in these strains. Expression from the PDC1 promoter in PDC1 wild-type and pdc1(0) strains was examined by the use of two reporter genes. Deletion of PDC1 led to increased expression of the two reporter genes regardless of whether the fusions were integrated into the genome or present on autonomously replicating plasmids. The results suggested that this effect was due to feedback regulation of the PDC1 promoter-driven expression in S. cerevisiae pdc1(0) strains. The yeast PDC1 gene was expressed in Escherichia coli, leading to an active PDC. This result shows that the PDC1-encoded subunit alone can form an active tetramer without yeast-specific processing steps.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Ammerer G., Hunter C. P., Rothman J. H., Saari G. C., Valls L. A., Stevens T. H. PEP4 gene of Saccharomyces cerevisiae encodes proteinase A, a vacuolar enzyme required for processing of vacuolar precursors. Mol Cell Biol. 1986 Jul;6(7):2490–2499. doi: 10.1128/mcb.6.7.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux A., Hess B. Allosteric properties of yeast pyruvate decarboxylase. FEBS Lett. 1970 Aug 31;9(5):293–296. doi: 10.1016/0014-5793(70)80381-7. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Butler G., McConnell D. J. Identification of an upstream activation site in the pyruvate decarboxylase structural gene (PDC1) of Saccharomyces cerevisiae. Curr Genet. 1988 Nov;14(5):405–412. doi: 10.1007/BF00521261. [DOI] [PubMed] [Google Scholar]
- Cohen R., Holland J. P., Yokoi T., Holland M. J. Identification of a regulatory region that mediates glucose-dependent induction of the Saccharomyces cerevisiae enolase gene ENO2. Mol Cell Biol. 1986 Jul;6(7):2287–2297. doi: 10.1128/mcb.6.7.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounaris A. D., Turkenkopf I., Buckwald S., Young A. Pyruvate decarboxylase. I. Protein dissociation into subunits under conditions in which thiamine pyrophosphate is released. J Biol Chem. 1971 Mar 10;246(5):1302–1309. [PubMed] [Google Scholar]
- Green J. B. Pyruvate decarboxylase is like acetolactate synthase (ILV2) and not like the pyruvate dehydrogenase E1 subunit. FEBS Lett. 1989 Mar 27;246(1-2):1–5. doi: 10.1016/0014-5793(89)80241-8. [DOI] [PubMed] [Google Scholar]
- Hopmann R. F. Hydroxyl-ion-induced subunit dissociation of east cytoplasmic pyruvate decarboxylase. A circular dichroism study. Eur J Biochem. 1980 Sep;110(1):311–318. doi: 10.1111/j.1432-1033.1980.tb04869.x. [DOI] [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
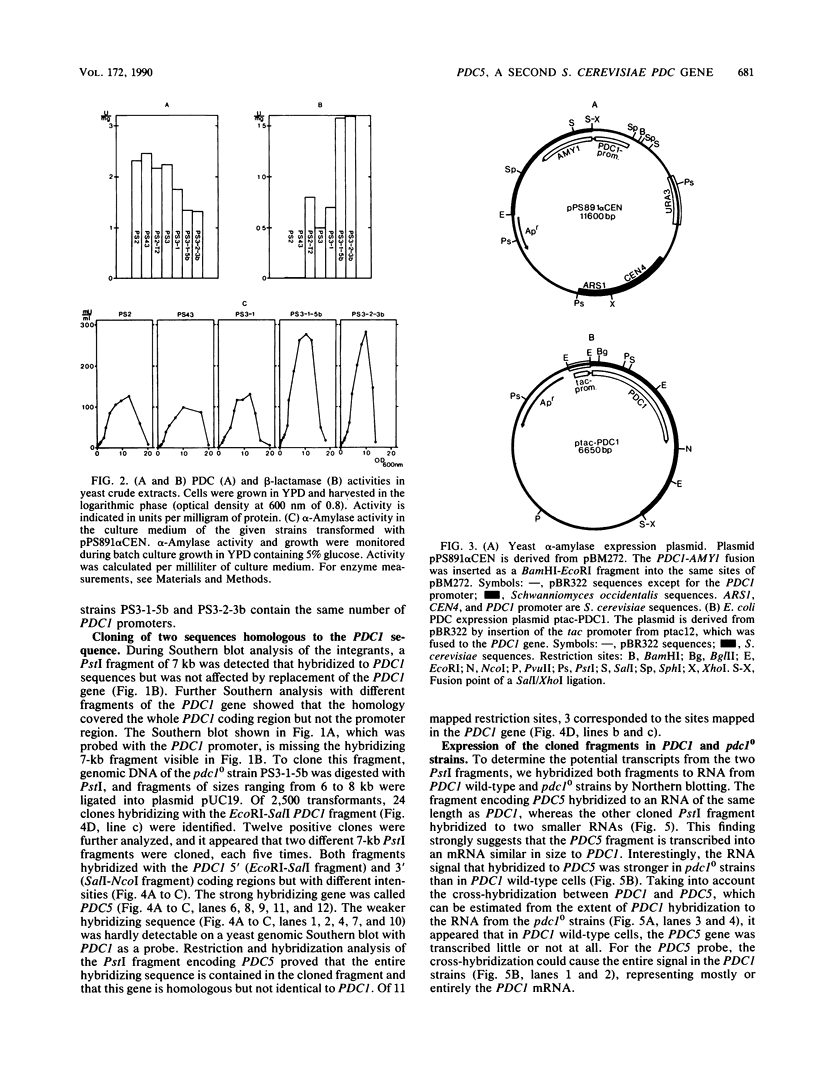
- Kellermann E., Hollenberg C. P. The glucose-and ethanol-dependent regulation of PDC1 from Saccharomyces cerevisiae are controlled by two distinct promoter regions. Curr Genet. 1988 Oct;14(4):337–344. doi: 10.1007/BF00419991. [DOI] [PubMed] [Google Scholar]
- Kellermann E., Seeboth P. G., Hollenberg C. P. Analysis of the primary structure and promoter function of a pyruvate decarboxylase gene (PDC1) from Saccharomyces cerevisiae. Nucleic Acids Res. 1986 Nov 25;14(22):8963–8977. doi: 10.1093/nar/14.22.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo D. J., Dikdan G., Jordan F. Resolution of brewers' yeast pyruvate decarboxylase into two isozymes. J Biol Chem. 1986 Mar 5;261(7):3316–3319. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludewig R., Schellenberger A. A new procedure to prepare highly purified and crystallized yeast pyruvate decarboxylase. FEBS Lett. 1974 Sep 1;45(1):340–343. doi: 10.1016/0014-5793(74)80876-8. [DOI] [PubMed] [Google Scholar]
- Rothman J. H., Hunter C. P., Valls L. A., Stevens T. H. Overproduction-induced mislocalization of a yeast vacuolar protein allows isolation of its structural gene. Proc Natl Acad Sci U S A. 1986 May;83(10):3248–3252. doi: 10.1073/pnas.83.10.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaff I., Green J. B., Gozalbo D., Hohmann S. A deletion of the PDC1 gene for pyruvate decarboxylase of yeast causes a different phenotype than previously isolated point mutations. Curr Genet. 1989 Feb;15(2):75–81. doi: 10.1007/BF00435452. [DOI] [PubMed] [Google Scholar]
- Schmitt H. D., Ciriacy M., Zimmermann F. K. The synthesis of yeast pyruvate decarboxylase is regulated by large variations in the messenger RNA level. Mol Gen Genet. 1983;192(1-2):247–252. doi: 10.1007/BF00327674. [DOI] [PubMed] [Google Scholar]
- Schmitt H. D., Zimmermann F. K. Genetic analysis of the pyruvate decarboxylase reaction in yeast glycolysis. J Bacteriol. 1982 Sep;151(3):1146–1152. doi: 10.1128/jb.151.3.1146-1152.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber M., König S., Hübner G., Schellenberger A. A rapid procedure for the preparation of highly purified pyruvate decarboxylase from brewer's yeast. Biomed Biochim Acta. 1983;42(4):343–349. [PubMed] [Google Scholar]
- Wright A. P., Png H. L., Hartley B. S. Identification, cloning and characterisation of a new gene required for full pyruvate decarboxylase activity in Saccharomyces cerevisiae. Curr Genet. 1989 Mar;15(3):171–175. doi: 10.1007/BF00435502. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zehender H., Trescher D., Ullrich J. Activity stain for pyruvate decarboxylase in polyacrylamide gels. Anal Biochem. 1983 Nov;135(1):16–21. doi: 10.1016/0003-2697(83)90724-8. [DOI] [PubMed] [Google Scholar]
- Zubenko G. S., Jones E. W. Protein degradation, meiosis and sporulation in proteinase-deficient mutants of Saccharomyces cerevisiae. Genetics. 1981 Jan;97(1):45–64. doi: 10.1093/genetics/97.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]