Abstract
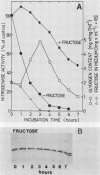
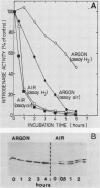
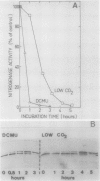
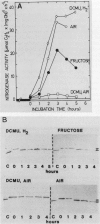
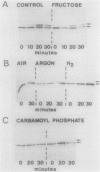
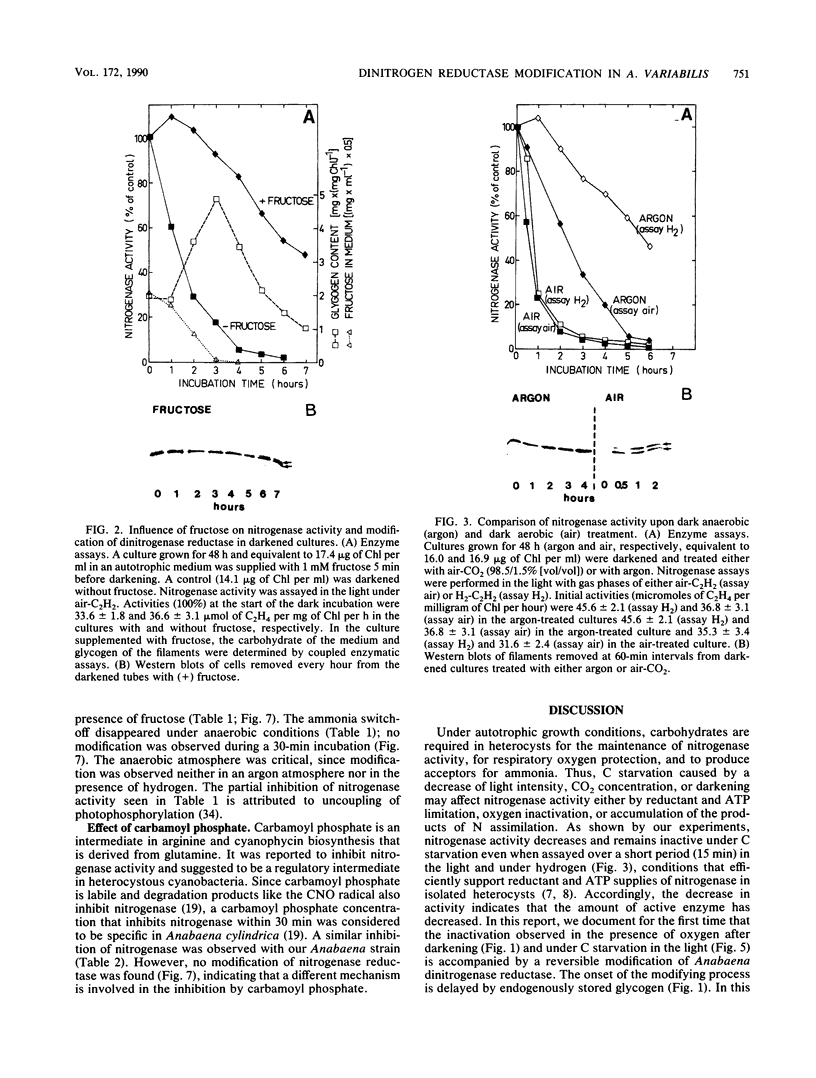
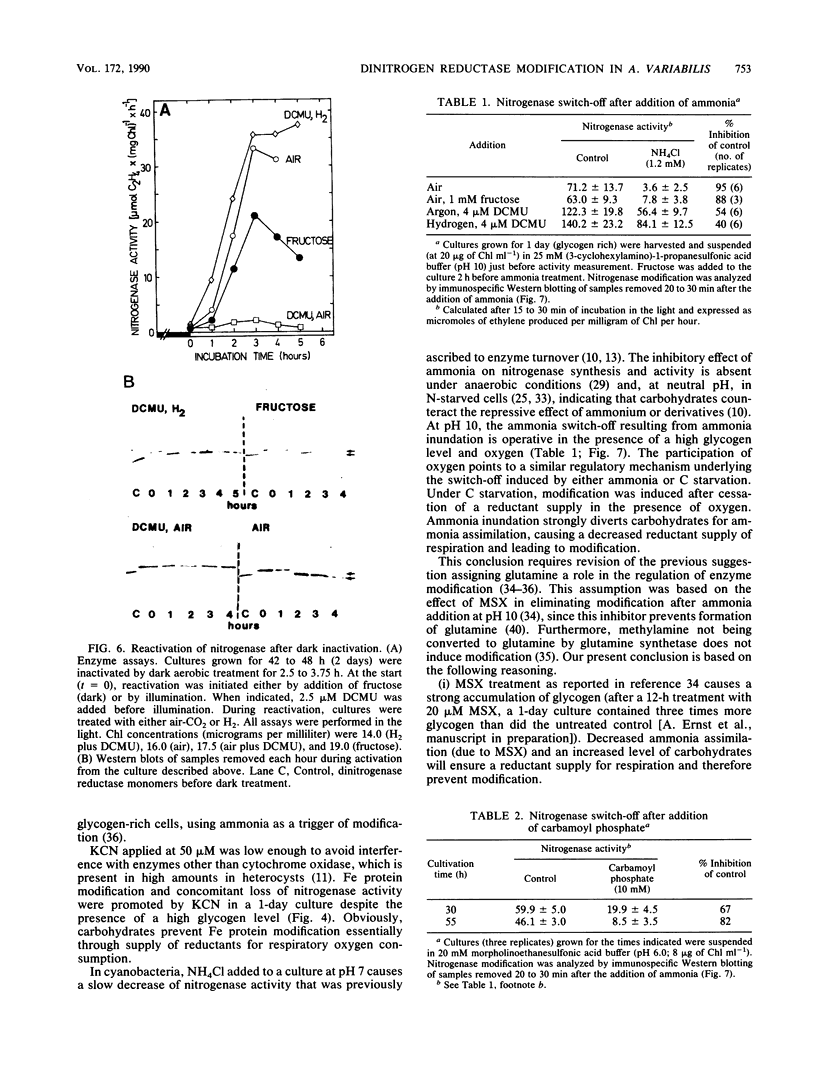
In the heterocystous cyanobacterium Anabaena variabilis, a change in nitrogenase activity and concomitant modification of dinitrogenase reductase (the Fe protein of nitrogenase) was induced either by NH4Cl at pH 10 (S. Reich and P. Böger, FEMS Microbiol. Lett. 58:81-86, 1989) or by cessation of C supply resulting from darkness, CO2 limitation, or inhibition of photosystem II activity. Modification induced by both C limitation and NH4Cl was efficiently prevented by anaerobic conditions. Under air, endogenously stored glycogen and added fructose protected against modification triggered by C limitation but not by NH4Cl. With stored glycogen present, dark modification took place after inhibition of respiration by KCN. Reactivation of inactivated nitrogenase and concomitant demodification of dinitrogenase reductase occurred after restoration of diazotrophic growth conditions. In previously C-limited cultures, reactivation was also observed in the dark after addition of fructose (heterotrophic growth) and under anaerobiosis upon reillumination in the presence of a photosynthesis inhibitor. The results indicate that modification of dinitrogenase reductase develops as a result of decreased carbohydrate-supported reductant supply of the heterocysts caused by C limitation or by increased diversion of carbohydrates towards ammonia assimilation. Apparently, a product of N assimilation such as glutamine is not necessary for modification. The increase of oxygen concentration in the heterocysts is a plausible consequence of all treatments causing Fe protein modification.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I., McSwain B. D., Tsujimoto H. Y., Wada K. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974 Aug 23;357(2):231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Bone D. H. Kinetics of synthesis of nitrogenase in batch and continuous culture of Anabaena flos-aquae. Arch Mikrobiol. 1971;80(3):242–251. doi: 10.1007/BF00410125. [DOI] [PubMed] [Google Scholar]
- Eidels L., Preiss J. Carbohydrate metabolism in Rhodopseudomonas capsulata: enzyme titers, glucose metabolism, and polyglucose polymer synthesis. Arch Biochem Biophys. 1970 Sep;140(1):75–89. doi: 10.1016/0003-9861(70)90011-1. [DOI] [PubMed] [Google Scholar]
- Helber J. T., Johnson T. R., Yarbrough L. R., Hirschberg R. Effect of nitrogenous compounds on nitrogenase gene expression in anaerobic cultures of Anabaena variabilis. J Bacteriol. 1988 Feb;170(2):558–563. doi: 10.1128/jb.170.2.558-563.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto R. H., Ludden P. W. Amino acid concentrations in Rhodospirillum rubrum during expression and switch-off of nitrogenase activity. J Bacteriol. 1987 Jul;169(7):3035–3043. doi: 10.1128/jb.169.7.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto R. H., Ludden P. W. Effect of ammonia, darkness, and phenazine methosulfate on whole-cell nitrogenase activity and Fe protein modification in Rhodospirillum rubrum. J Bacteriol. 1984 May;158(2):713–720. doi: 10.1128/jb.158.2.713-720.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrie A. C. Effect of carbamoyl phosphate on nitrogenase in Anabaena cylindrica Lemm. J Bacteriol. 1979 Jul;139(1):115–119. doi: 10.1128/jb.139.1.115-119.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. D., Hu C. Z., Yoch D. C. Changes in amino acid and nucleotide pools of Rhodospirillum rubrum during switch-off of nitrogenase activity initiated by NH4+ or darkness. J Bacteriol. 1987 Jan;169(1):231–237. doi: 10.1128/jb.169.1.231-237.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery R. G., Ludden P. W. Purification and properties of dinitrogenase reductase ADP-ribosyltransferase from the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem. 1988 Nov 15;263(32):16714–16719. [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Activating factor for the iron protein of nitrogenase from Rhodospirillum rubrum. Science. 1976 Oct 22;194(4263):424–426. doi: 10.1126/science.824729. [DOI] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Purification and properties of nitrogenase from Rhodospirillum rubrum, and evidence for phosphate, ribose and an adenine-like unit covalently bound to the iron protein. Biochem J. 1978 Oct 1;175(1):251–259. doi: 10.1042/bj1750251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry M. A., Horne A. J., Benemann J. R. Physiological Studies of Oxygen Protection Mechanisms in the Heterocysts of Anabaena cylindrica. Appl Environ Microbiol. 1984 Mar;47(3):449–454. doi: 10.1128/aem.47.3.449-454.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A. H., Nordlund S. Regulation of nitrogenase synthesis in intact cells of Rhodospirillum rubrum: inactivation of nitrogen fixation by ammonia, L-glutamine and L-asparagine. J Gen Microbiol. 1975 Nov;91(1):53–62. doi: 10.1099/00221287-91-1-53. [DOI] [PubMed] [Google Scholar]
- Pope M. R., Murrell S. A., Ludden P. W. Covalent modification of the iron protein of nitrogenase from Rhodospirillum rubrum by adenosine diphosphoribosylation of a specific arginine residue. Proc Natl Acad Sci U S A. 1985 May;82(10):3173–3177. doi: 10.1073/pnas.82.10.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J. Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol. 1984;38:419–458. doi: 10.1146/annurev.mi.38.100184.002223. [DOI] [PubMed] [Google Scholar]
- Preston G. G., Ludden P. W. Change in subunit composition of the iron protein of nitrogenase from Rhodospirillum rubrum during activation and inactivation of iron protein. Biochem J. 1982 Sep 1;205(3):489–494. doi: 10.1042/bj2050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari L. L., Triplett E. W., Ludden P. W. Purification and properties of the activating enzyme for iron protein of nitrogenase from the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem. 1984 Dec 25;259(24):15502–15508. [PubMed] [Google Scholar]
- Smith R. L., Van Baalen C., Tabita F. R. Alteration of the Fe protein of nitrogenase by oxygen in the cyanobacterium Anabaena sp. strain CA. J Bacteriol. 1987 Jun;169(6):2537–2542. doi: 10.1128/jb.169.6.2537-2542.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. D., Rowell P. Effects of L-methionine-DL-sulphoximine on the assimilation of newly fixed NH3, acetylene reduction and heterocyst production in Anabaena cylindrica. Biochem Biophys Res Commun. 1975 Aug 4;65(3):846–856. doi: 10.1016/s0006-291x(75)80463-3. [DOI] [PubMed] [Google Scholar]
- Vignais P. M., Colbeau A., Willison J. C., Jouanneau Y. Hydrogenase, nitrogenase, and hydrogen metabolism in the photosynthetic bacteria. Adv Microb Physiol. 1985;26:155–234. doi: 10.1016/s0065-2911(08)60397-5. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Cantu M. Changes in the regulatory form of Rhodospirillum rubrum nitrogenase as influenced by nutritional and environmental factors. J Bacteriol. 1980 Jun;142(3):899–907. doi: 10.1128/jb.142.3.899-907.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Gotto J. W. Effect of light intensity and inhibitors of nitrogen assimilation on NH4+ inhibition of nitrogenase activity in Rhodospirillum rubrum and Anabaena sp. J Bacteriol. 1982 Aug;151(2):800–806. doi: 10.1128/jb.151.2.800-806.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft W. G., Castillo F. Regulatory properties of the nitrogenase from Rhodopseudomonas palustris. Arch Microbiol. 1978 Apr 27;117(1):53–60. doi: 10.1007/BF00689351. [DOI] [PubMed] [Google Scholar]