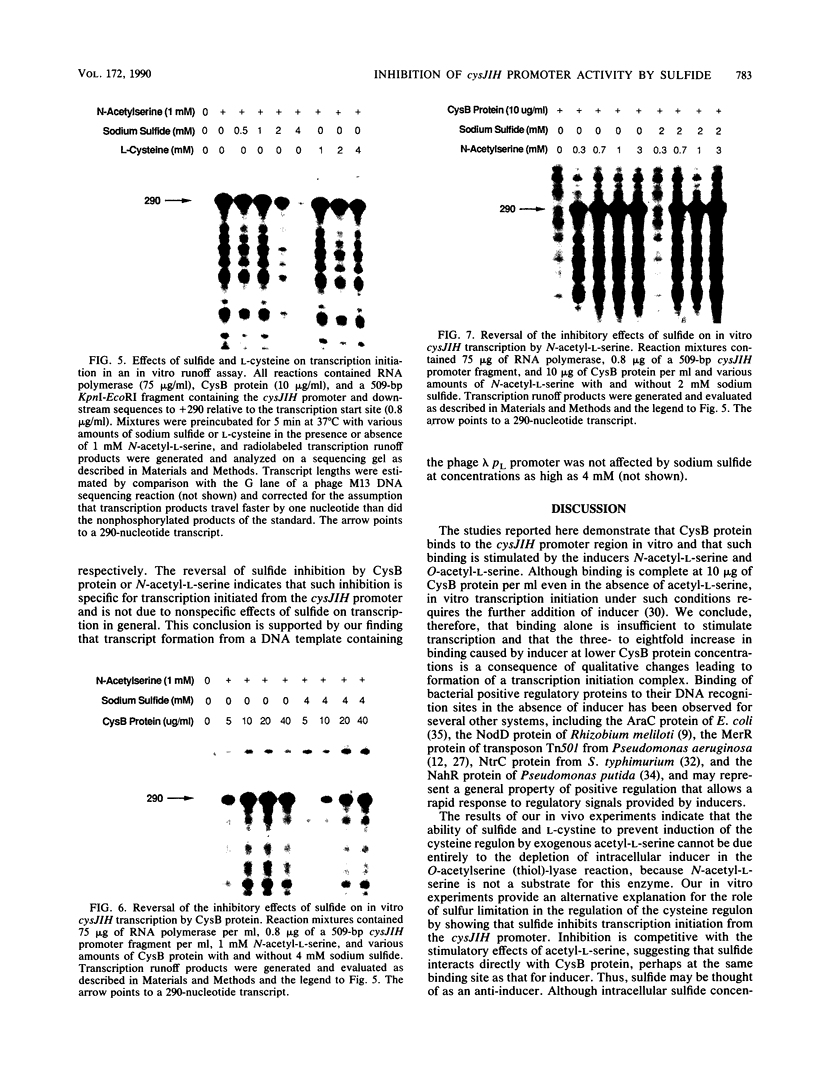
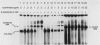Abstract
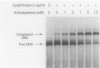
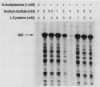
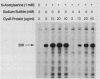
The cysteine regulon of Salmonella typhimurium is positively regulated by the CysB protein and an inducer, which can be either O-acetyl-L-serine or N-acetyl-L-serine. In vivo experiments confirmed that sulfide and L-cysteine (supplied as L-cystine) interfere with induction by exogenously supplied O-acetyl-L-serine and also showed the same effects when N-acetyl-L-serine was used as an inducer. In a gel shift assay, purified CysB protein bound specifically to a 278-base-pair DNA fragment containing the S. typhimurium cysJIH promoter region. Binding occurred in the absence of inducer but did not stimulate in vitro transcription initiation, indicating that binding alone is insufficient to cause formation of a transcription initiation complex. Addition of N-acetyl-L-serine or O-acetyl-L-serine was required for transcription initiation and also stimulated binding three- to eightfold. Sulfide inhibited both transcription initiation and binding by interfering with the stimulatory effects of inducer in a competitive manner. These findings indicate that sulfide is an anti-inducer and may explain why full expression of the cysteine regulon requires sulfur limitation. L-Cysteine did not affect in vitro transcription initiation or binding of CysB protein to the cysJIH promoter region. The in vivo effects of L-cysteine may be secondary to its degradation to sulfide by the inducible enzyme cysteine desulfhydrase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker M. A., Kredich N. M., Tomkins G. M. The purification and characterization of O-acetylserine sulfhydrylase-A from Salmonella typhimurium. J Biol Chem. 1969 May 10;244(9):2418–2427. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brennan R. G., Matthews B. W. The helix-turn-helix DNA binding motif. J Biol Chem. 1989 Feb 5;264(4):1903–1906. [PubMed] [Google Scholar]
- Byrne C. R., Monroe R. S., Ward K. A., Kredich N. M. DNA sequences of the cysK regions of Salmonella typhimurium and Escherichia coli and linkage of the cysK regions to ptsH. J Bacteriol. 1988 Jul;170(7):3150–3157. doi: 10.1128/jb.170.7.3150-3157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMEREC M., GILLESPIE D. H., MIZOBUCHI K. GENETIC STRUCTURE OF THE CYST REGION OF THE SALMONELLA GENOME. Genetics. 1963 Aug;48:997–1009. doi: 10.1093/genetics/48.8.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Fisher R. F., Egelhoff T. T., Mulligan J. T., Long S. R. Specific binding of proteins from Rhizobium meliloti cell-free extracts containing NodD to DNA sequences upstream of inducible nodulation genes. Genes Dev. 1988 Mar;2(3):282–293. doi: 10.1101/gad.2.3.282. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heltzel A., Gambill D., Jackson W. J., Totis P. A., Summers A. O. Overexpression and DNA-binding properties of the mer-encoded regulatory protein from plasmid NR1 (Tn21). J Bacteriol. 1987 Jul;169(7):3379–3384. doi: 10.1128/jb.169.7.3379-3384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Haughn G. W., Calvo J. M., Wallace J. C. A large family of bacterial activator proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6602–6606. doi: 10.1073/pnas.85.18.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulanicka M. D., Hallquist S. G., Kredich N. M., Mojica-A T. Regulation of O-acetylserine sulfhydrylase B by L-cysteine in Salmonella typhimurium. J Bacteriol. 1979 Oct;140(1):141–146. doi: 10.1128/jb.140.1.141-146.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C. L., Colless V., Smith M. T., Molasky D. O., Malo M. S., Loughlin R. E. Lambda transducing phage and clones carrying genes of the cysJIHDC gene cluster of Escherichia coli K12. J Gen Microbiol. 1987 Oct;133(10):2707–2717. doi: 10.1099/00221287-133-10-2707. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Positive control of sulphate reduction in Escherichia coli. The nature of the pleiotropic cysteineless mutants of E. coli K12. Biochem J. 1968 Dec;110(3):597–602. doi: 10.1042/bj1100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Wheldrake J. F., Pasternak C. A. The control of sulphate reduction in Escherichia coli by O-acetyl-L-serine. Biochem J. 1968 Mar;107(1):51–53. doi: 10.1042/bj1070051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M., Becker M. A., Tomkins G. M. Purification and characterization of cysteine synthetase, a bifunctional protein complex, from Salmonella typhimurium. J Biol Chem. 1969 May 10;244(9):2428–2439. [PubMed] [Google Scholar]
- Kredich N. M., Keenan B. S., Foote L. J. The purification and subunit structure of cysteine desulfhydrase from Salmonella typhimurium. J Biol Chem. 1972 Nov 25;247(22):7157–7162. [PubMed] [Google Scholar]
- Kredich N. M. Regulation of L-cysteine biosynthesis in Salmonella typhimurium. I. Effects of growth of varying sulfur sources and O-acetyl-L-serine on gene expression. J Biol Chem. 1971 Jun 10;246(11):3474–3484. [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- Loughlin R. E. Polarity of the cysJIH operon of Salmonella typhimurium. J Gen Microbiol. 1975 Feb;86(2):275–282. doi: 10.1099/00221287-86-2-275. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- NARITA K. Isolation of acetylseryltyrosine from the chymotryptic digests of proteins of five strains of tobacco mosaic virus. Biochim Biophys Acta. 1958 Nov;30(2):352–359. doi: 10.1016/0006-3002(58)90060-x. [DOI] [PubMed] [Google Scholar]
- O'Halloran T., Walsh C. Metalloregulatory DNA-binding protein encoded by the merR gene: isolation and characterization. Science. 1987 Jan 9;235(4785):211–214. doi: 10.1126/science.3798107. [DOI] [PubMed] [Google Scholar]
- Ostrowski J., Barber M. J., Rueger D. C., Miller B. E., Siegel L. M., Kredich N. M. Characterization of the flavoprotein moieties of NADPH-sulfite reductase from Salmonella typhimurium and Escherichia coli. Physicochemical and catalytic properties, amino acid sequence deduced from DNA sequence of cysJ, and comparison with NADPH-cytochrome P-450 reductase. J Biol Chem. 1989 Sep 25;264(27):15796–15808. [PubMed] [Google Scholar]
- Ostrowski J., Hulanicka D. Constitutive mutation of cysJIH operon in a cysB deletion strain of Salmonella typhimurium. Mol Gen Genet. 1979 Sep;175(2):145–149. doi: 10.1007/BF00425530. [DOI] [PubMed] [Google Scholar]
- Ostrowski J., Jagura-Burdzy G., Kredich N. M. DNA sequences of the cysB regions of Salmonella typhimurium and Escherichia coli. J Biol Chem. 1987 May 5;262(13):5999–6005. [PubMed] [Google Scholar]
- Ostrowski J., Kredich N. M. Molecular characterization of the cysJIH promoters of Salmonella typhimurium and Escherichia coli: regulation by cysB protein and N-acetyl-L-serine. J Bacteriol. 1989 Jan;171(1):130–140. doi: 10.1128/jb.171.1.130-140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski J., Wu J. Y., Rueger D. C., Miller B. E., Siegel L. M., Kredich N. M. Characterization of the cysJIH regions of Salmonella typhimurium and Escherichia coli B. DNA sequences of cysI and cysH and a model for the siroheme-Fe4S4 active center of sulfite reductase hemoprotein based on amino acid homology with spinach nitrite reductase. J Biol Chem. 1989 Sep 15;264(26):15726–15737. [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Popham D. L., Szeto D., Keener J., Kustu S. Function of a bacterial activator protein that binds to transcriptional enhancers. Science. 1989 Feb 3;243(4891):629–635. doi: 10.1126/science.2563595. [DOI] [PubMed] [Google Scholar]
- Schell M. A., Poser E. F. Demonstration, characterization, and mutational analysis of NahR protein binding to nah and sal promoters. J Bacteriol. 1989 Feb;171(2):837–846. doi: 10.1128/jb.171.2.837-846.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Davis P. S., Kamin H. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. 3. The Escherichia coli hemoflavoprotein: catalytic parameters and the sequence of electron flow. J Biol Chem. 1974 Mar 10;249(5):1572–1586. [PubMed] [Google Scholar]
- Siegel L. M., Davis P. S. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. IV. The Escherichia coli hemoflavoprotein: subunit structure and dissociation into hemoprotein and flavoprotein components. J Biol Chem. 1974 Mar 10;249(5):1587–1598. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wheldrake J. F. Intracellular concentration of cysteine in Escherichia coli and its relation to repression of the sulphate-activating enzymes. Biochem J. 1967 Nov;105(2):697–699. doi: 10.1042/bj1050697. [DOI] [PMC free article] [PubMed] [Google Scholar]