Abstract
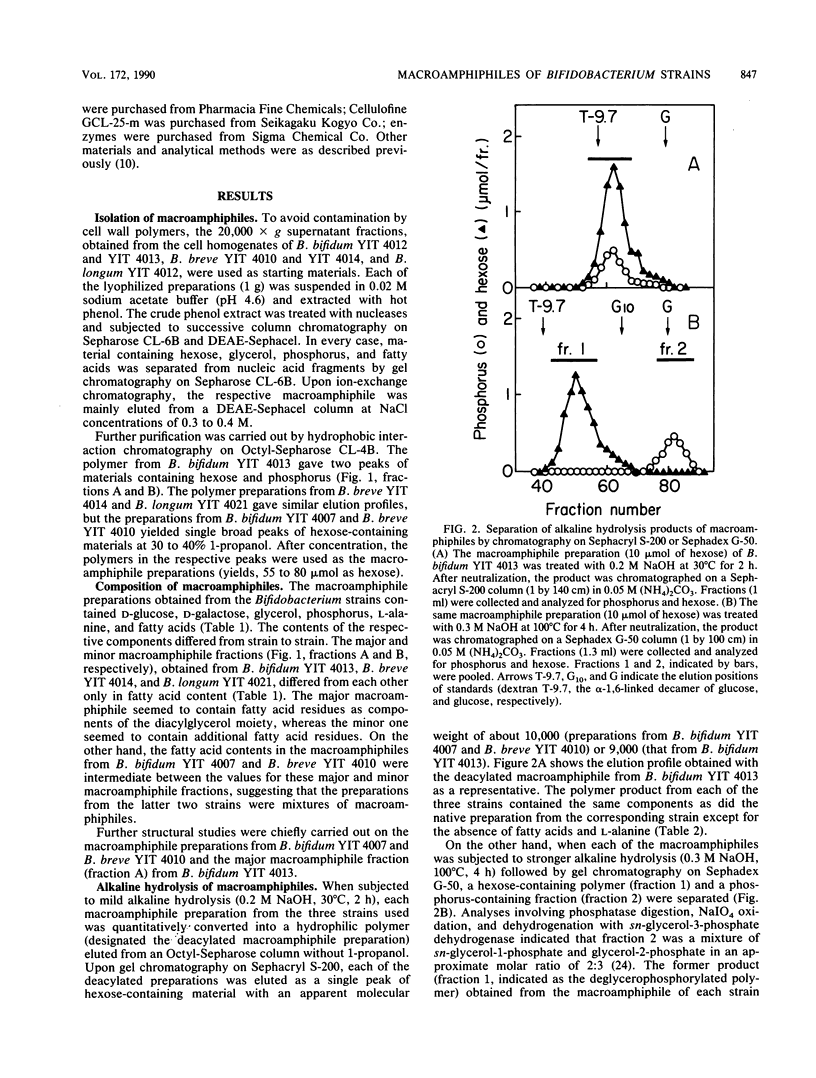
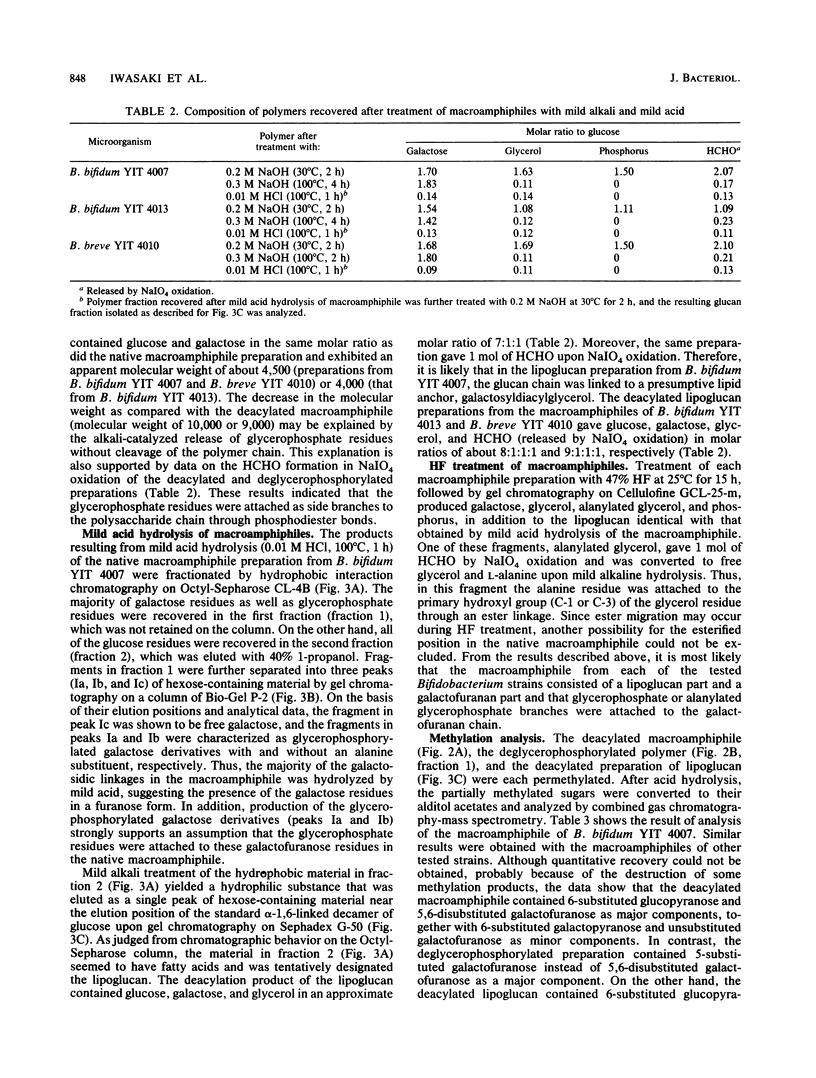
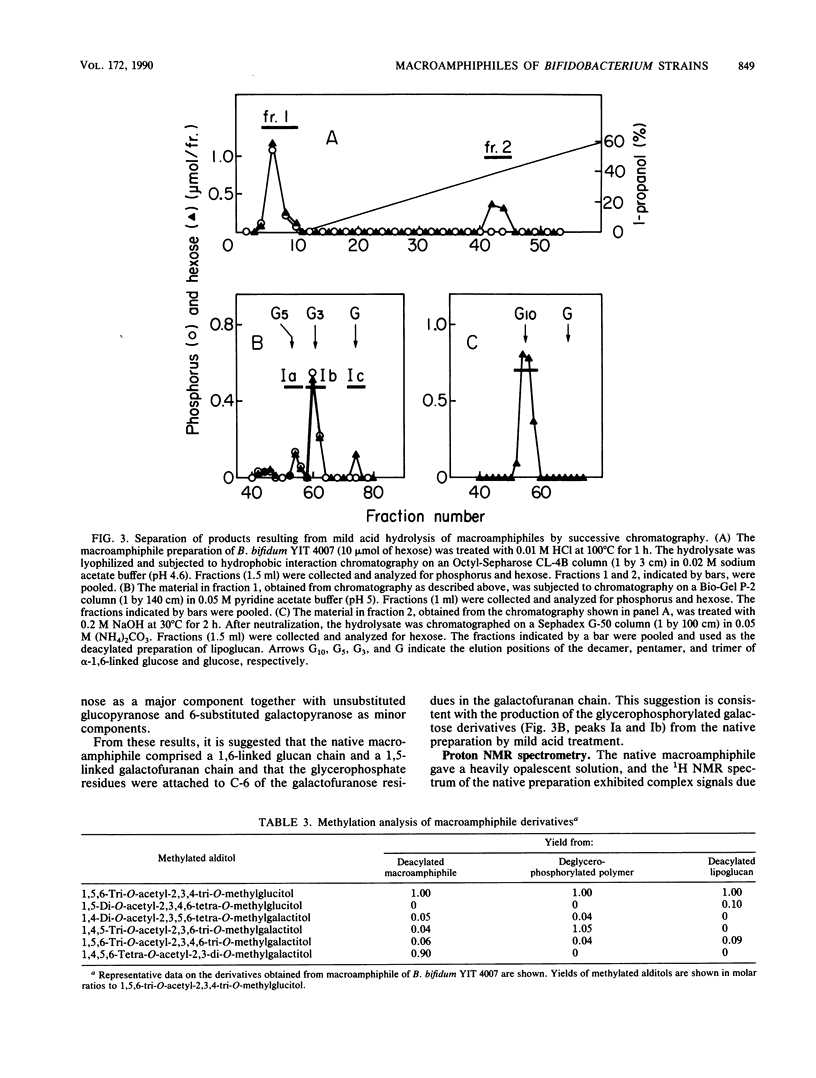
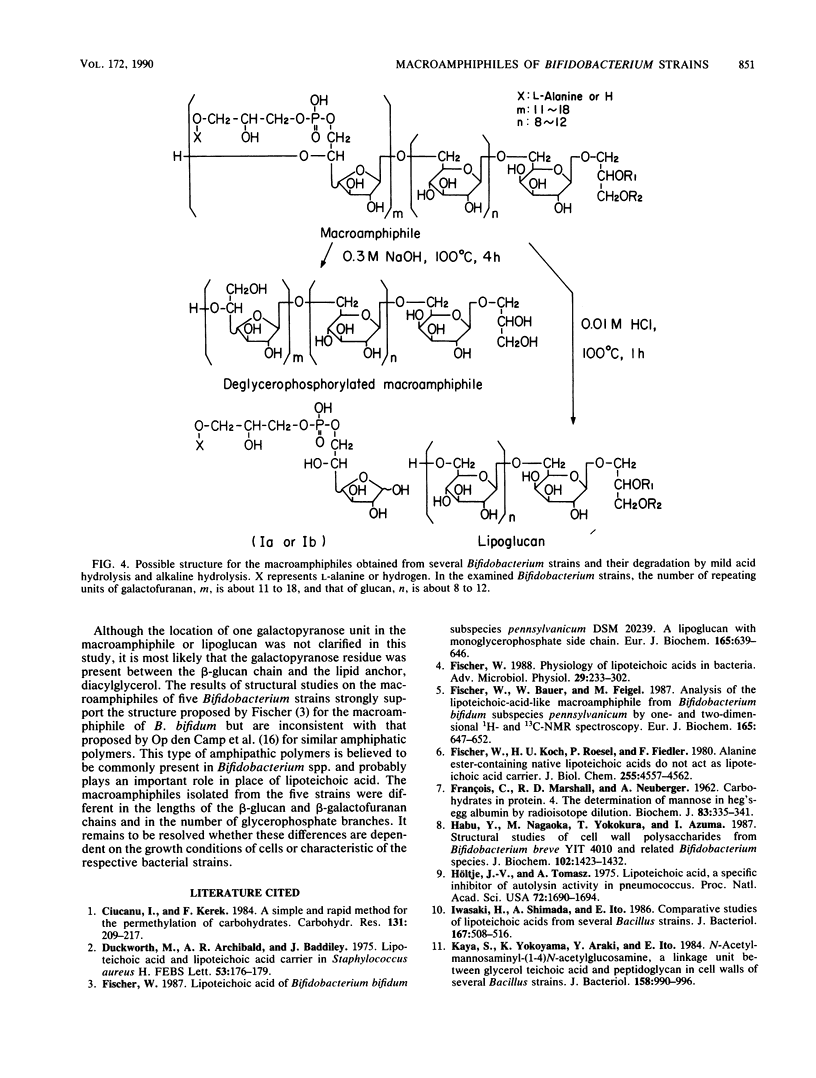
Lipoteichoic acid-like substances, macroamphiphiles, were isolated from cell homogenates of Bifidobacterium bifidum YIT 4007 and YIT 4013, Bifidobacterium breve YIT 4010 and YIT 4014, and Bifidobacterium longum YIT 4021 by phenol extraction followed by nuclease digestion, gel chromatography, ion-exchange chromatography, and hydrophobic interaction chromatography. The macroamphiphile preparations from these five strains contained D-glucose, D-galactose, glycerol, phosphorus, L-alanine, and fatty acids in molar ratios of 1.00, 1.57 to 1.95, 1.02 to 1.99, 0.97 to 1.72, 0.15 to 0.46, and 0.16 to 0.43. Data from structural analyses including methylation, 1H nuclear magnetic resonance measurement, alkaline hydrolysis, mild acid hydrolysis, and hydrogen fluoride treatment led to the most likely common structure for the macroamphiphiles of the examined strains, (formula; see text) where Gro-P is glycerophosphate, m is the number of repeating units of galactofuranan, and n is the number of repeating units of glucan. Whereas the polymers from the respective strains differed in the numbers of repeating units of the galactofuranan and glucan moieties and in the number of fatty acid residues, the proposed structure is essentially the same as that reported previously for the macroamphiphile of B. bifidum subsp. pennsylvanicum DSM 20239 by W. Fischer (Eur. J. Biochem. 165:639-646, 1987).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duckworth M., Archibald A. R., Baddiley J. Lipoteichoic acid and lipoteichoic acid carrier in Staphylococcus aureus H. FEBS Lett. 1975 May 1;53(2):176–179. doi: 10.1016/0014-5793(75)80013-5. [DOI] [PubMed] [Google Scholar]
- FRANCOIS C., MARSHALL R. D., NEUBERGER A. Carbohydrates in protein. 4. The determination of mannose in hen's-egg albumin by radioisotope dilution. Biochem J. 1962 May;83:335–341. doi: 10.1042/bj0830335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W. 'Lipoteichoic acid' of Bifidobacterium bifidum subspecies pennsylvanicum DSM 20239. A lipoglycan with monoglycerophosphate side chains. Eur J Biochem. 1987 Jun 15;165(3):639–646. doi: 10.1111/j.1432-1033.1987.tb11488.x. [DOI] [PubMed] [Google Scholar]
- Fischer W., Bauer W., Feigel M. Analysis of the lipoteichoic-acid-like macroamphiphile from Bifidobacterium bifidum subspecies pennsylvanicum by one- and two-dimensional 1H- and 13C-NMR spectroscopy. Eur J Biochem. 1987 Jun 15;165(3):647–652. doi: 10.1111/j.1432-1033.1987.tb11489.x. [DOI] [PubMed] [Google Scholar]
- Fischer W., Koch H. U., Rösel P., Fiedler F. Alanine ester-containing native lipoteichoic acids do not act as lipoteichoic acid carrier. Isolation, structural and functional characterization. J Biol Chem. 1980 May 25;255(10):4557–4562. [PubMed] [Google Scholar]
- Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- Habu Y., Nagaoka M., Yokokura T., Azuma I. Structural studies of cell wall polysaccharides from Bifidobacterium breve YIT 4010 and related Bifidobacterium species. J Biochem. 1987 Dec;102(6):1423–1432. doi: 10.1093/oxfordjournals.jbchem.a122188. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H., Shimada A., Ito E. Comparative studies of lipoteichoic acids from several Bacillus strains. J Bacteriol. 1986 Aug;167(2):508–516. doi: 10.1128/jb.167.2.508-516.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya S., Yokoyama K., Araki Y., Ito E. N-acetylmannosaminyl(1----4)N-acetylglucosamine, a linkage unit between glycerol teichoic acid and peptidoglycan in cell walls of several Bacillus strains. J Bacteriol. 1984 Jun;158(3):990–996. doi: 10.1128/jb.158.3.990-996.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Lambert P. A., Hancock I. C., Baddiley J. Occurrence and function of membrane teichoic acids. Biochim Biophys Acta. 1977 May 31;472(1):1–12. doi: 10.1016/0304-4157(77)90012-0. [DOI] [PubMed] [Google Scholar]
- Lambert P. A., Hancock I. C., Baddiley J. The interaction of magnesium ions with teichoic acid. Biochem J. 1975 Sep;149(3):519–524. doi: 10.1042/bj1490519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op den Camp H. J., Peeters P. A., Oosterhof A., Veerkamp J. H. Immunochemical studies on the lipoteichoic acids of Bifidobacterium bifidum subsp. pennsylvanicum. J Gen Microbiol. 1985 Mar;131(3):661–668. doi: 10.1099/00221287-131-3-661. [DOI] [PubMed] [Google Scholar]
- Op den Camp H. J., Veerkamp J. H., Oosterhof A., Van Halbeek H. Structure of the lipoteichoic acids from Bifidobacterium bifidum spp. pennsylvanicum. Biochim Biophys Acta. 1984 Sep 12;795(2):301–313. doi: 10.1016/0005-2760(84)90080-8. [DOI] [PubMed] [Google Scholar]
- Pless D. D., Schmit A. S., Lennarz W. J. The characterization of mannan of Micrococcus lysodeikticus as an acidic lipopolysaccharide. J Biol Chem. 1975 Feb 25;250(4):1319–1327. [PubMed] [Google Scholar]
- Powell D. A., Duckworth M., Baddiley J. A membrane-associated lipomannan in micrococci. Biochem J. 1975 Nov;151(2):387–397. doi: 10.1042/bj1510387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toon P., Brown P. E., Baddiley J. The lipid-teichoic acid complex in the cytoplasmic membrane of Streptococcus faecalis N.C.I.B. 8191. Biochem J. 1972 Apr;127(2):399–409. doi: 10.1042/bj1270399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Broady K. W., Evans J. D., Knox K. W. New cellular and extracellular amphipathic antigen from Actinomyces viscosus NY1. Infect Immun. 1978 Nov;22(2):615–616. doi: 10.1128/iai.22.2.615-616.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Studies on the group F antigen of lactobacilli: isolation of a teichoic acid-lipid complex from Lactobacillus fermenti NCTC 6991. J Gen Microbiol. 1970 Mar;60(3):293–301. doi: 10.1099/00221287-60-3-293. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Koga T., Mizuno J., Hamada S. Chemical and immunological characterization of a novel amphipathic antigen from biotype B Streptococcus sanguis. J Gen Microbiol. 1985 Aug;131(8):1981–1988. doi: 10.1099/00221287-131-8-1981. [DOI] [PubMed] [Google Scholar]
- Yoneyama T., Araki Y., Ito E. The primary structure of teichuronic acid in Bacillus subtilis AHU 1031. Eur J Biochem. 1984 May 15;141(1):83–89. doi: 10.1111/j.1432-1033.1984.tb08160.x. [DOI] [PubMed] [Google Scholar]


