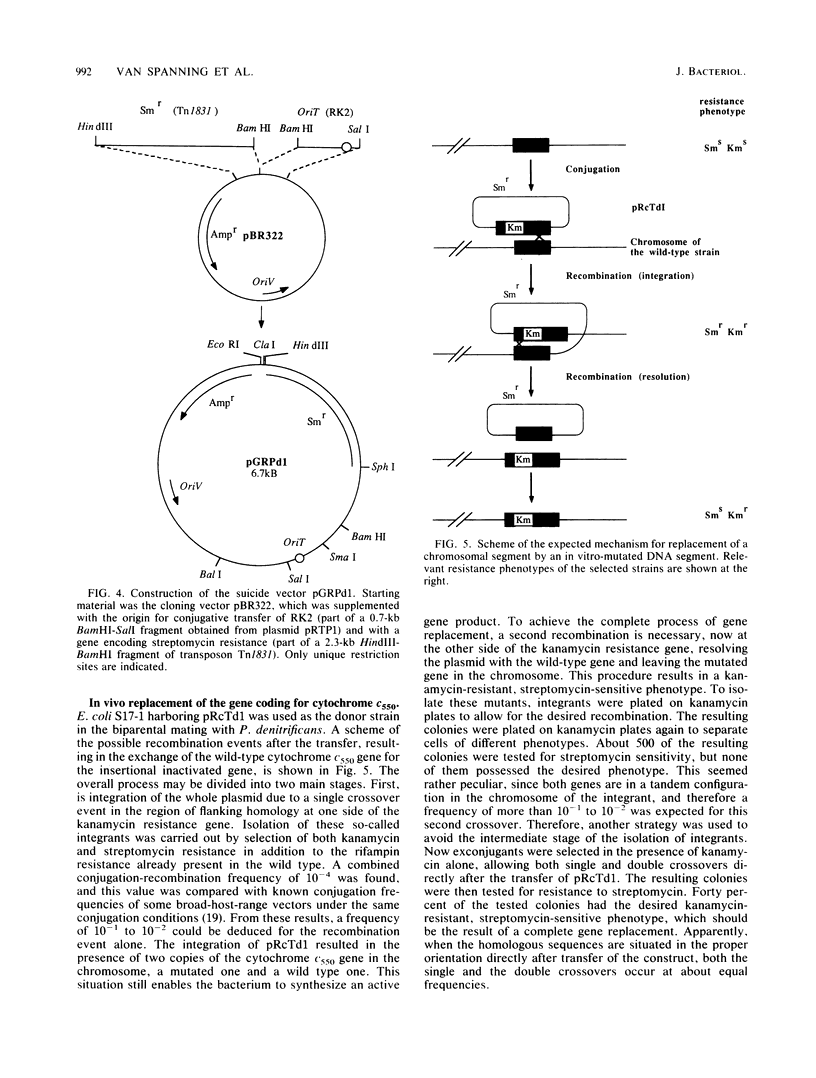
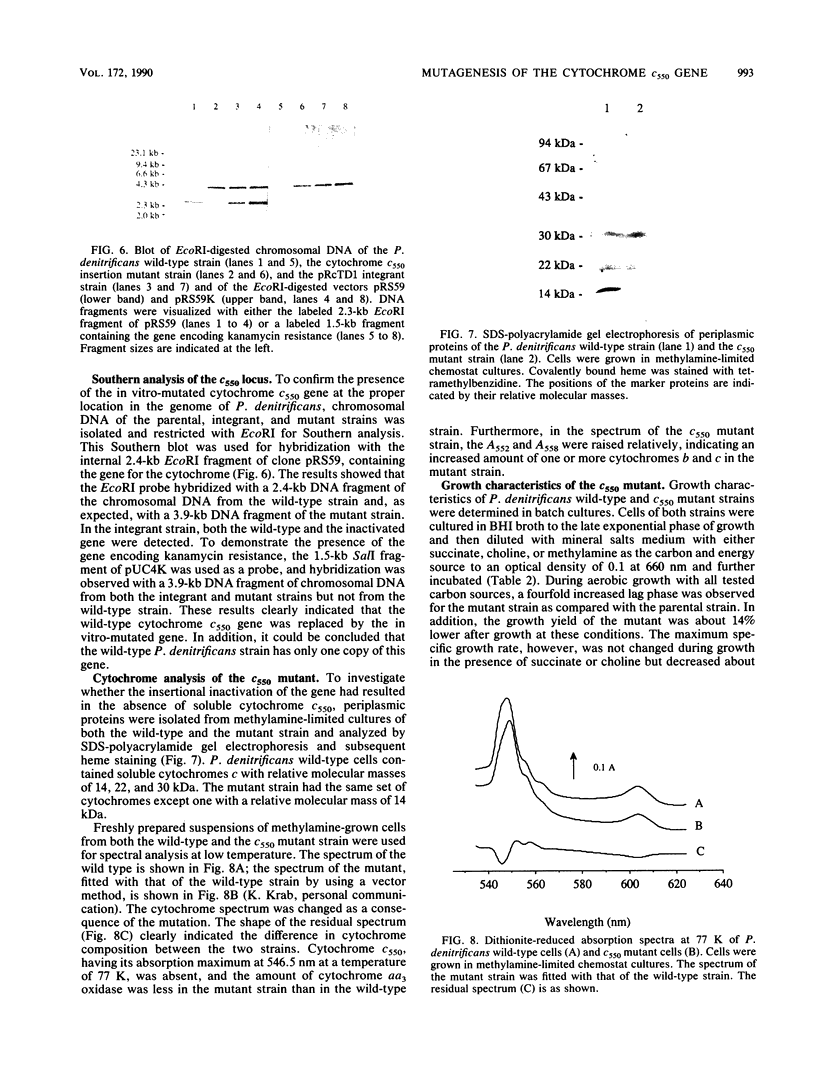
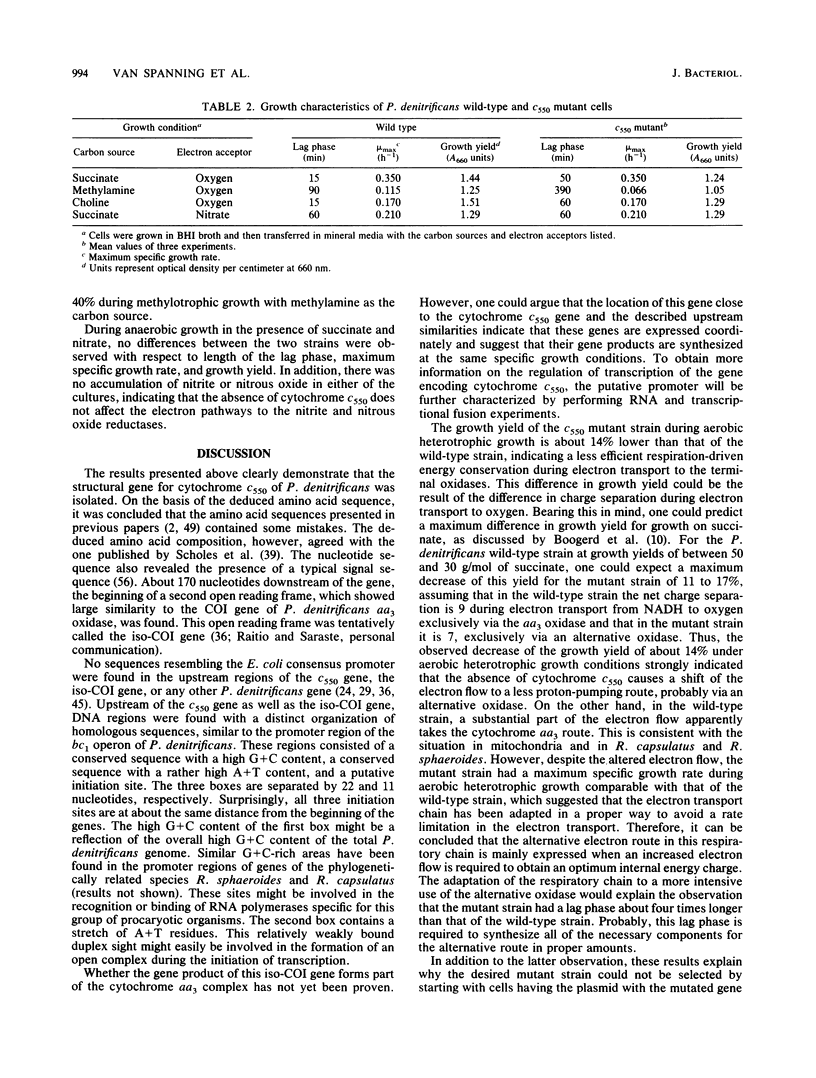
Abstract
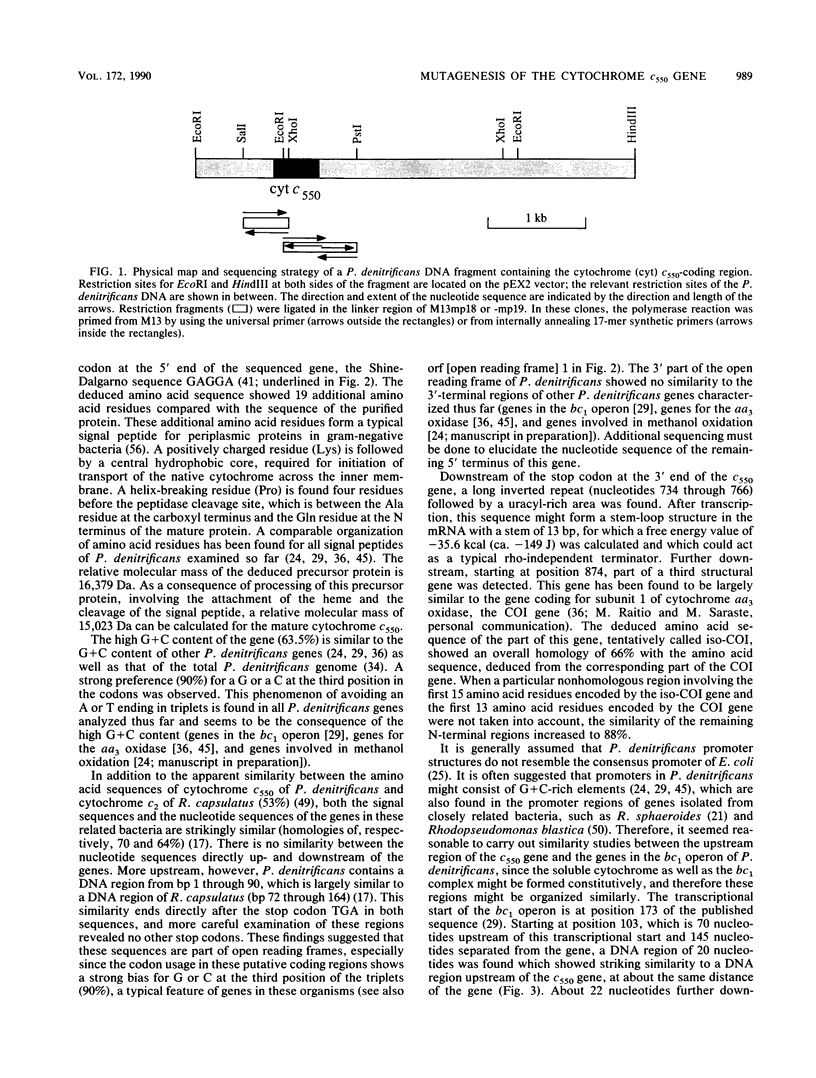
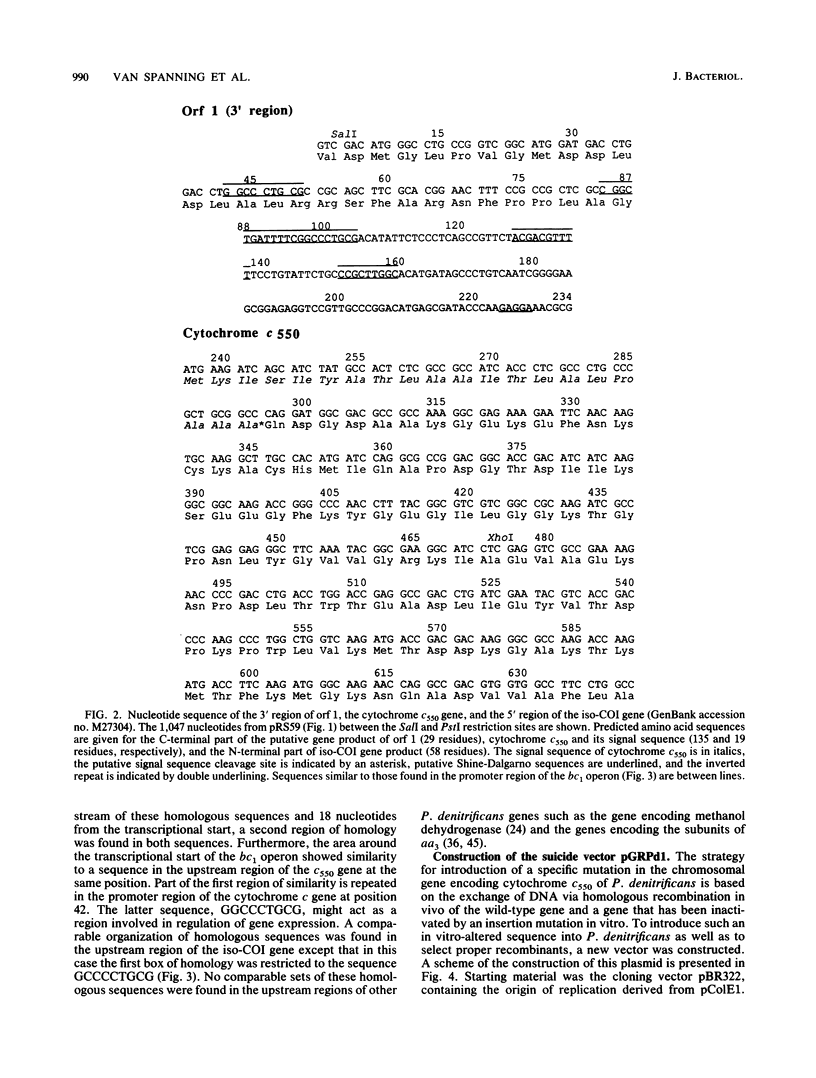
By using synthetic oligonucleotides, the gene encoding soluble cytochrome c550 was isolated from a genomic bank of Paracoccus denitrificans. The nucleotide sequence of the gene was determined, and the deduced amino acid sequence of the mature protein was found to be similar to the primary structure of purified cytochrome c550 except for the presence of seven additional amino acid residues at the C terminus. At the N terminus of the primary structure was found an additional stretch of 19 amino acid residues that had the typical features of the signal sequence of the cytochrome. Comparison of the nucleotide sequences of the upstream regions of the P. denitrificans cytochrome c550 gene and bc1 operon revealed three regions with a distinct organization that showed strong similarity. Downstream of the c550 gene was found part of another gene, the deduced amino acid sequence of which showed strong homology with subunit 1 of the cytochrome aa3 oxidase. For gene replacement experiments, the suicide vector pGRPd1 was constructed. The cytochrome c550 gene was inactivated by insertion of a kanamycin resistance gene, and the mutated gene was cloned into this vector. Recombination with the wild-type gene resulted in a mutant strain with an inactivated cytochrome gene. Isolated mutant strains were unable to synthesize the soluble cytochrome, as judged by spectrum analysis and analysis of periplasmic proteins by gel electrophoresis and heme staining. The mutation resulted in a 14% decrease in the growth yield during aerobic heterotrophic growth and in a 40% decrease in the maximum specific growth rate during growth on methylamine. Furthermore, a longer lag phase was observed under both growth conditions. The mutation had no effect on growth yield, maximum specific growth rate, and duration of the lag phase during anaerobic growth in the presence of nitrate. In addition, there was no accumulation of nitrite and nitrous oxide.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albracht S. P., van Verseveld H. W., Hagen W. R., Kalkman M. L. A comparison of the respiratory chain in particles from Paracoccus denitrificans and bovine heart mitochondria by EPR spectroscopy. Biochim Biophys Acta. 1980 Dec 3;593(2):173–186. doi: 10.1016/0005-2728(80)90055-9. [DOI] [PubMed] [Google Scholar]
- Ambler R. P., Meyer T. E., Kamen M. D., Schichman S. A., Sawyer L. A reassessment of the structure of Paracoccus cytochrome c-550. J Mol Biol. 1981 Apr 5;147(2):351–356. doi: 10.1016/0022-2836(81)90445-9. [DOI] [PubMed] [Google Scholar]
- Anthony C. The microbial metabolism of C1 compounds. The cytochromes of Pseudomaonas AM1. Biochem J. 1975 Feb;146(2):289–298. doi: 10.1042/bj1460289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage J. P., Ingham C., Evans M. C. Role of proton motive force in phototactic and aerotactic responses of Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Mar;161(3):967–972. doi: 10.1128/jb.161.3.967-972.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarini-Melandri A., Jones O. T., Hauska G. Cytochrome c2--an electron carrier shared by the respiratory and photosynthetic electron transport chain of Rhodopseudomonas capsulata. FEBS Lett. 1978 Feb 15;86(2):151–154. doi: 10.1016/0014-5793(78)80551-1. [DOI] [PubMed] [Google Scholar]
- Berry E. A., Trumpower B. L. Isolation of ubiquinol oxidase from Paracoccus denitrificans and resolution into cytochrome bc1 and cytochrome c-aa3 complexes. J Biol Chem. 1985 Feb 25;260(4):2458–2467. [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bosma G., Braster M., Stouthamer A. H., van Verseveld H. W. Isolation and characterization of ubiquinol oxidase complexes from Paracoccus denitrificans cells cultured under various limiting growth conditions in the chemostat. Eur J Biochem. 1987 Jun 15;165(3):657–663. doi: 10.1111/j.1432-1033.1987.tb11491.x. [DOI] [PubMed] [Google Scholar]
- Bosma G., Braster M., Stouthamer A. H., van Verseveld H. W. Subfractionation and characterization of soluble c-type cytochromes from Paracoccus denitrificans cultured under various limiting conditions in the chemostat. Eur J Biochem. 1987 Jun 15;165(3):665–670. doi: 10.1111/j.1432-1033.1987.tb11492.x. [DOI] [PubMed] [Google Scholar]
- CHANG J. P., MORRIS J. G. Studies on the utilization of nitrate by Micrococcus denitrificans. J Gen Microbiol. 1962 Oct;29:301–310. doi: 10.1099/00221287-29-2-301. [DOI] [PubMed] [Google Scholar]
- Chandrasekar R., Klapper M. H. Methylamine dehydrogenase and cytochrome c552 from the bacterium W3A1. J Biol Chem. 1986 Mar 15;261(8):3616–3619. [PubMed] [Google Scholar]
- Cox J. C., Ingledew W. J., Haddock B. A., Lawford H. G. The variable cytochrome content of Paracoccus denitrificans grown aerobically under different conditions. FEBS Lett. 1978 Sep 15;93(2):261–265. doi: 10.1016/0014-5793(78)81117-x. [DOI] [PubMed] [Google Scholar]
- Cox R. B., Quayle J. R. The autotrophic growth of Micrococcus denitrificans on Methanol. Biochem J. 1975 Sep;150(3):569–571. doi: 10.1042/bj1500569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldal F., Cheng S., Applebaum J., Davidson E., Prince R. C. Cytochrome c(2) is not essential for photosynthetic growth of Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2012–2016. doi: 10.1073/pnas.83.7.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson V. L., Kumar M. A. Cytochrome c-550 mediates electron transfer from inducible periplasmic c-type cytochromes to the cytoplasmic membrane of Paracoccus denitrificans. FEBS Lett. 1989 Mar 13;245(1-2):271–273. doi: 10.1016/0014-5793(89)80235-2. [DOI] [PubMed] [Google Scholar]
- Gabellini N., Sebald W. Nucleotide sequence and transcription of the fbc operon from Rhodopseudomonas sphaeroides. Evaluation of the deduced amino acid sequences of the FeS protein, cytochrome b and cytochrome c1. Eur J Biochem. 1986 Feb 3;154(3):569–579. doi: 10.1111/j.1432-1033.1986.tb09437.x. [DOI] [PubMed] [Google Scholar]
- Gennis R. B., Casey R. P., Azzi A., Ludwig B. Purification and characterization of the cytochrome c oxidase from Rhodopseudomonas sphaeroides. Eur J Biochem. 1982 Jun 15;125(1):189–195. doi: 10.1111/j.1432-1033.1982.tb06667.x. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms N., de Vries G. E., Maurer K., Hoogendijk J., Stouthamer A. H. Isolation and nucleotide sequence of the methanol dehydrogenase structural gene from Paracoccus denitrificans. J Bacteriol. 1987 Sep;169(9):3969–3975. doi: 10.1128/jb.169.9.3969-3975.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M., Davidson V. L. Purification and properties of methylamine dehydrogenase from Paracoccus denitrificans. J Bacteriol. 1987 Apr;169(4):1712–1717. doi: 10.1128/jb.169.4.1712-1717.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John P., Whatley F. R. Paracoccus denitrificans and the evolutionary origin of the mitochondrion. Nature. 1975 Apr 10;254(5500):495–498. doi: 10.1038/254495a0. [DOI] [PubMed] [Google Scholar]
- Kuo L. M., Davies H. C., Smith L. Monoclonal antibodies to cytochrome c from Paracoccus denitrificans: effects on electron transport reactions. Biochim Biophys Acta. 1985 Oct 9;809(3):388–395. doi: 10.1016/0005-2728(85)90189-6. [DOI] [PubMed] [Google Scholar]
- Kurowski B., Ludwig B. The genes of the Paracoccus denitrificans bc1 complex. Nucleotide sequence and homologies between bacterial and mitochondrial subunits. J Biol Chem. 1987 Oct 5;262(28):13805–13811. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Margalit R., Schejter A. Cytochrome c: a thermodynamic study of the relationships among oxidation state, ion-binding and structural parameters. 1. The effects of temperature, pH and electrostatic media on the standard redox potential of cytochrome c. Eur J Biochem. 1973 Feb 1;32(3):492–499. doi: 10.1111/j.1432-1033.1973.tb02633.x. [DOI] [PubMed] [Google Scholar]
- Mermod N., Lehrbach P. R., Reineke W., Timmis K. N. Transcription of the TOL plasmid toluate catabolic pathway operon of Pseudomonas putida is determined by a pair of co-ordinately and positively regulated overlapping promoters. EMBO J. 1984 Nov;3(11):2461–2466. doi: 10.1002/j.1460-2075.1984.tb02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Raitio M., Jalli T., Saraste M. Isolation and analysis of the genes for cytochrome c oxidase in Paracoccus denitrificans. EMBO J. 1987 Sep;6(9):2825–2833. doi: 10.1002/j.1460-2075.1987.tb02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes P. B., McLain G., Smith L. Purification and properties of a c-type cytochrome from Micrococcus denitrificans. Biochemistry. 1971 May 25;10(11):2072–2076. doi: 10.1021/bi00787a017. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinrücke P., Steffens G. C., Panskus G., Buse G., Ludwig B. Subunit II of cytochrome c oxidase from Paracoccus denitrificans. DNA sequence, gene expression and the protein. Eur J Biochem. 1987 Sep 15;167(3):431–439. doi: 10.1111/j.1432-1033.1987.tb13356.x. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Black W., Falkow S. The construction of a cloning vector designed for gene replacement in Bordetella pertussis. Gene. 1986;50(1-3):133–140. doi: 10.1016/0378-1119(86)90318-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Timkovich R., Dickerson R. E., Margoliash E. Amino acid sequence of Paracoccus denitrificans cytochrome c550. J Biol Chem. 1976 Apr 25;251(8):2197–2206. [PubMed] [Google Scholar]
- Timkovich R., Dickerson R. E. The structure of Paracoccus denitrificans cytochrome c550. J Biol Chem. 1976 Jul 10;251(13):4033–4046. doi: 10.2210/pdb155c/pdb. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Falk G., Walker J. E. Rhodopseudomonas blastica atp operon. Nucleotide sequence and transcription. J Mol Biol. 1984 Oct 25;179(2):185–214. doi: 10.1016/0022-2836(84)90465-0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witholt B., Heerikhuizen H. V., De Leij L. How does lysozyme penetrate through the bacterial outer membrane? Biochim Biophys Acta. 1976 Sep 7;443(3):534–544. doi: 10.1016/0005-2736(76)90471-5. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Embden J., Cohen S. N. Molecular and genetic studies of an R factor system consisting of independent transfer and drug resistance plasmids. J Bacteriol. 1973 Nov;116(2):699–709. doi: 10.1128/jb.116.2.699-709.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Verseveld H. W., Bosma G. The respiratory chain and energy conservation in the mitochondrion-like bacterium Paracoccus denitrificans. Microbiol Sci. 1987 Nov;4(11):329–333. [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]