Abstract
Important protein-based immunoreactivities have long been associated with the cell wall core of mycobacteria. In order to explore the molecular basis of such activities, purified cell walls of Mycobacterium tuberculosis were extracted with sodium dodecyl sulfate to produce an insoluble residue composed of the mycolylarabinogalactan-peptidoglycan complex and about 2% of unextractable protein. Treatment of the product from an avirulent strain of M. tuberculosis with trifluoromethanesulfonic acid released a single polypeptide with a molecular size of 23 kilodaltons, accounting for all of the insoluble cell wall protein. Extensive purification and then analysis of the 23-kilodalton protein demonstrated the absence of diaminopimelic acid, muramic acid, or other peptidoglycan components, pointing to either a novel linkage between protein and peptidoglycan or a noncovalent but tenacious association. The released 23-kilodalton protein showed amino acid homology and other similarities to the outer membrane protein OmpF of Escherichia coli. Although a similar product was released in small quantities from cell walls of the virulent M. tuberculosis Erdman and H37Rv by lysozyme treatment, the cell walls of virulent bacilli were dominated by the presence of poly-alpha-L-glutamine, accounting for as much as 10% of their weight. The poly-alpha-L-glutamine was successfully separated from the cell wall proper, demonstrating again the absence of a covalent association between peptidoglycan and the polymer. The antigenicity of these products is demonstrated, and their roles vis-a-vis analogous polypeptides from other bacteria in immunogenicity, pathogenicity, and bacterial physiology are discussed.
Full text
PDF




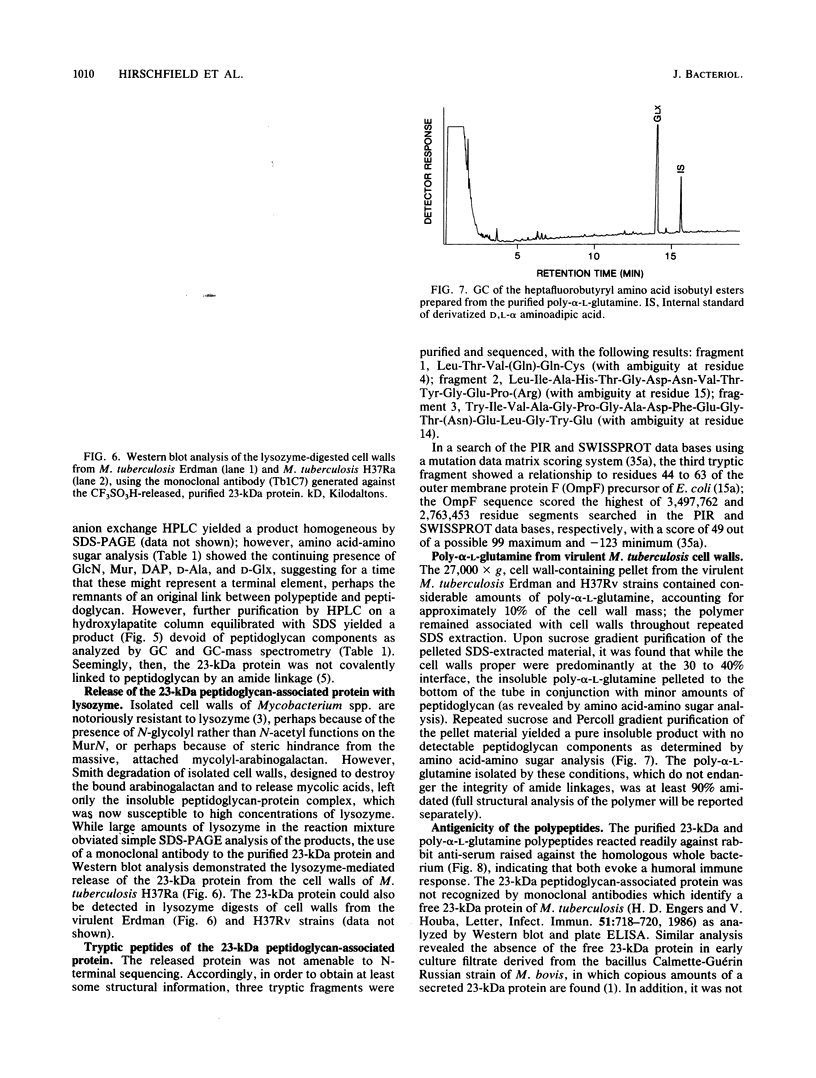
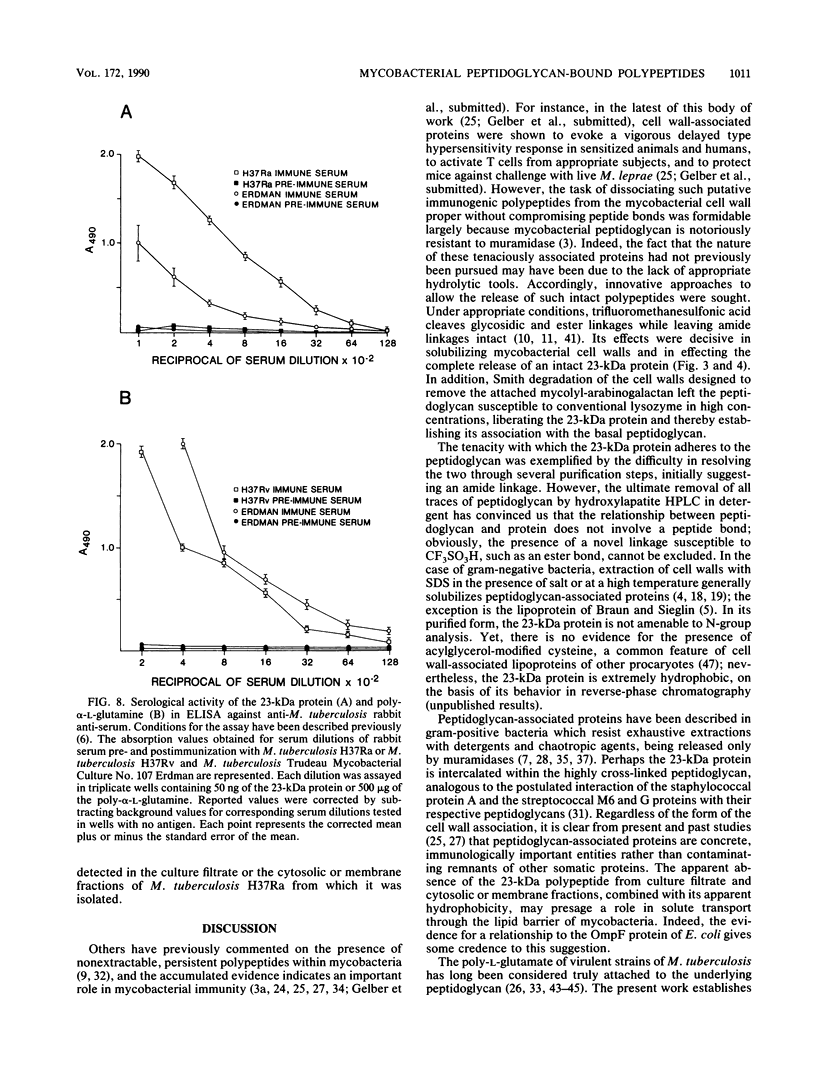


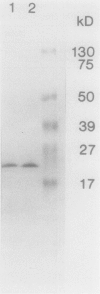
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Smith I., Grange J., Steele J., Rook G. Subdivision of daughter strains of bacille Calmette-Guérin (BCG) according to secreted protein patterns. J Gen Microbiol. 1986 Nov;132(11):3047–3053. doi: 10.1099/00221287-132-11-3047. [DOI] [PubMed] [Google Scholar]
- Anacker R. L., Barclay W. R., Brehmer W., Goode G., List R. H., Ribi E., Tarmina D. F. Effectiveness of cell walls of Mycobacterium bovis strain BCG administered by various routes and in different adjuvants in protecting mice against airborne infection with Mycobacterium tuberculosis strain H37Rv. Am Rev Respir Dis. 1969 Feb;99(2):242–248. doi: 10.1164/arrd.1969.99.2.242. [DOI] [PubMed] [Google Scholar]
- Azuma I., Thomas D. W., Adam A., Ghuysen J. M., Bonaly R., Petit J. F., Lederer E. Occurrence of N-glycolylmuramic acid in bacterial cell walls. A preliminary survey. Biochim Biophys Acta. 1970 Jun;208(3):444–451. doi: 10.1016/0304-4165(70)90217-5. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Mehra V., Hirschfield G. R., Fong S. J., Abou-Zeid C., Rook G. A., Hunter S. W., Brennan P. J., Modlin R. L. Characterization of T cell antigens associated with the cell wall protein-peptidoglycan complex of Mycobacterium tuberculosis. J Immunol. 1989 Oct 15;143(8):2656–2662. [PubMed] [Google Scholar]
- Benz R. Structure and function of porins from gram-negative bacteria. Annu Rev Microbiol. 1988;42:359–393. doi: 10.1146/annurev.mi.42.100188.002043. [DOI] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee D., Bozic C. M., Knisley C., Cho S. N., Brennan P. J. Phenolic glycolipids of Mycobacterium bovis: new structures and synthesis of a corresponding seroreactive neoglycoprotein. Infect Immun. 1989 Feb;57(2):322–330. doi: 10.1128/iai.57.2.322-330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J., Streips U. N., Fan V. S., Brown W. C., Mobley H., Mansfield J. M. Cell wall protein in Bacillus subtilis. J Bacteriol. 1977 Jan;129(1):547–549. doi: 10.1128/jb.129.1.547-549.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper P. The walls of Mycobacterium lepraemurium: chemistry and ultrastructure. J Gen Microbiol. 1971 Dec;69(3):313–324. doi: 10.1099/00221287-69-3-313. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Spiro R. G. Selective deglycosylation of the heparan sulfate proteoglycan of bovine glomerular basement membrane and identification of the core protein. J Biol Chem. 1987 May 15;262(14):6893–6898. [PubMed] [Google Scholar]
- Fiscus S. A., Teramoto Y. A., Mildbrand M. M., Knisley C. V., Winston S. E., Pedersen N. C. Competitive enzyme immunoassays for the rapid detection of antibodies to feline infectious peritonitis virus polypeptides. J Clin Microbiol. 1985 Sep;22(3):395–401. doi: 10.1128/jcm.22.3.395-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., McNeil M., Modlin R. L., Mehra V., Bloom B. R., Brennan P. J. Isolation and characterization of the highly immunogenic cell wall-associated protein of Mycobacterium leprae. J Immunol. 1989 Apr 15;142(8):2864–2872. [PubMed] [Google Scholar]
- Inokuchi K., Mutoh N., Matsuyama S., Mizushima S. Primary structure of the ompF gene that codes for a major outer membrane protein of Escherichia coli K-12. Nucleic Acids Res. 1982 Nov 11;10(21):6957–6968. doi: 10.1093/nar/10.21.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefford M. J. Macrophage activation and resistance to pulmonary tuberculosis. Infect Immun. 1980 May;28(2):508–515. doi: 10.1128/iai.28.2.508-515.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Bronstein H., van Selm N., Peters R. Peptidoglycan-associated outer membrane proteins in gammegatine bacteria. Biochim Biophys Acta. 1977 Mar 17;465(3):571–578. doi: 10.1016/0005-2736(77)90274-7. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- MacKenzie S. L., Hogge L. R. Gas chromatography-mass spectrometry of the N(O)-heptafluorobutyryl isobutyl esters of the protein amino acids using electron impact ionisation. J Chromatogr. 1977 Feb 21;132(3):485–493. doi: 10.1016/s0021-9673(00)82912-x. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. Resistance to intracellular infection. J Infect Dis. 1971 Apr;123(4):439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- Marianne T., Perini J. M., Houvenaghel M. C., Tramu G., Lamblin G., Roussel P. Action of trifluoromethanesulfonic acid on highly glycosylated regions of human bronchial mucins. Carbohydr Res. 1986 Aug 15;151:7–19. doi: 10.1016/s0008-6215(00)90325-2. [DOI] [PubMed] [Google Scholar]
- McNeil M., Tsang A. Y., Brennan P. J. Structure and antigenicity of the specific oligosaccharide hapten from the glycopeptidolipid antigen of Mycobacterium avium serotype 4, the dominant Mycobacterium isolated from patients with acquired immune deficiency syndrome. J Biol Chem. 1987 Feb 25;262(6):2630–2635. [PubMed] [Google Scholar]
- Mehra V., Bloom B. R., Torigian V. K., Mandich D., Reichel M., Young S. M., Salgame P., Convit J., Hunter S. W., McNeil M. Characterization of Mycobacterium leprae cell wall-associated proteins with the use of T lymphocyte clones. J Immunol. 1989 Apr 15;142(8):2873–2878. [PubMed] [Google Scholar]
- Melancon-Kaplan J., Hunter S. W., McNeil M., Stewart C., Modlin R. L., Rea T. H., Convit J., Salgame P., Mehra V., Bloom B. R. Immunological significance of Mycobacterium leprae cell walls. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1917–1921. doi: 10.1073/pnas.85.6.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore D., Acharya N. P., Jollès P. Caractérisation de quantités importantes d'acide glutamique dans les parois de mycobactéries de souches humaines virulentes. C R Acad Sci Hebd Seances Acad Sci D. 1966 Sep 12;263(11):846–848. [PubMed] [Google Scholar]
- Misaki A., Yukawa S., Tsuchiya K., Yamasaki T. Studies on cell walls of Mycobacteria. I. Chemical and biological properties of the cell walls and the mucopeptide of BCG. J Biochem. 1966 Apr;59(4):388–396. doi: 10.1093/oxfordjournals.jbchem.a128314. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Isolation and characterization of the cell-associated region of group A streptococcal M6 protein. J Bacteriol. 1988 Jun;170(6):2618–2624. doi: 10.1128/jb.170.6.2618-2624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiet P. H., Wietzerbin J., Zissman E., Petit J. F., Lederer E. Analysis of the cell wall of five strains of Myocbacterium tuberculosis BCG and of an attenuated human strain, W 115. Infect Immun. 1976 Mar;13(3):677–681. doi: 10.1128/iai.13.3.677-681.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi E., Granger D. L., Milner K. C., Yamamoto K., Strain S. M., Parker R., Smith R. W., Brehmer W., Azuma I. Induction of resistance to tuberculosis in mice with defined components of mycobacteria and with some unrelated materials. Immunology. 1982 Jun;46(2):297–305. [PMC free article] [PubMed] [Google Scholar]
- Russell R. R. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- Shively J. M. Inclusion bodies of prokaryotes. Annu Rev Microbiol. 1974;28(0):167–187. doi: 10.1146/annurev.mi.28.100174.001123. [DOI] [PubMed] [Google Scholar]
- Sjöquist J., Movitz J., Johansson I. B., Hjelm H. Localization of protein A in the bacteria. Eur J Biochem. 1972 Oct 17;30(1):190–194. doi: 10.1111/j.1432-1033.1972.tb02086.x. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Supplement on future research in tuberculosis. Prospects and priorities for elimination. Endorsement of the American Thoracic Society Board of Directors in March 1986. Am Rev Respir Dis. 1986 Aug;134(2):401–423. [PubMed] [Google Scholar]
- Takayama K., Schnoes H. K., Armstrong E. L., Boyle R. W. Site of inhibitory action of isoniazid in the synthesis of mycolic acids in Mycobacterium tuberculosis. J Lipid Res. 1975 Jul;16(4):308–317. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietzerbin-Falszpan J., Das B. C., Gros C., Petit J. F., Lederer E. The amino acids of the cell wall of Mycobacterium tuberculosis var. bovis, strain BCG. Presence of a poly(L-glutamic acid). Eur J Biochem. 1973 Feb 1;32(3):525–532. doi: 10.1111/j.1432-1033.1973.tb02637.x. [DOI] [PubMed] [Google Scholar]
- Wietzerbin J., Lederer F., Petit J. F. Structural study of the poly-l-Glutamic acid of the cell wall of Mycobacterium tuberculosis var hominis, strain Brevannes. Biochem Biophys Res Commun. 1975 Jan 20;62(2):246–252. doi: 10.1016/s0006-291x(75)80130-6. [DOI] [PubMed] [Google Scholar]
- Wong M. Y., Steck P. A., Gray G. R. The major mycolic acids of Mycobacterium smegmatis. Characterization of their homologous series. J Biol Chem. 1979 Jul 10;254(13):5734–5740. [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]