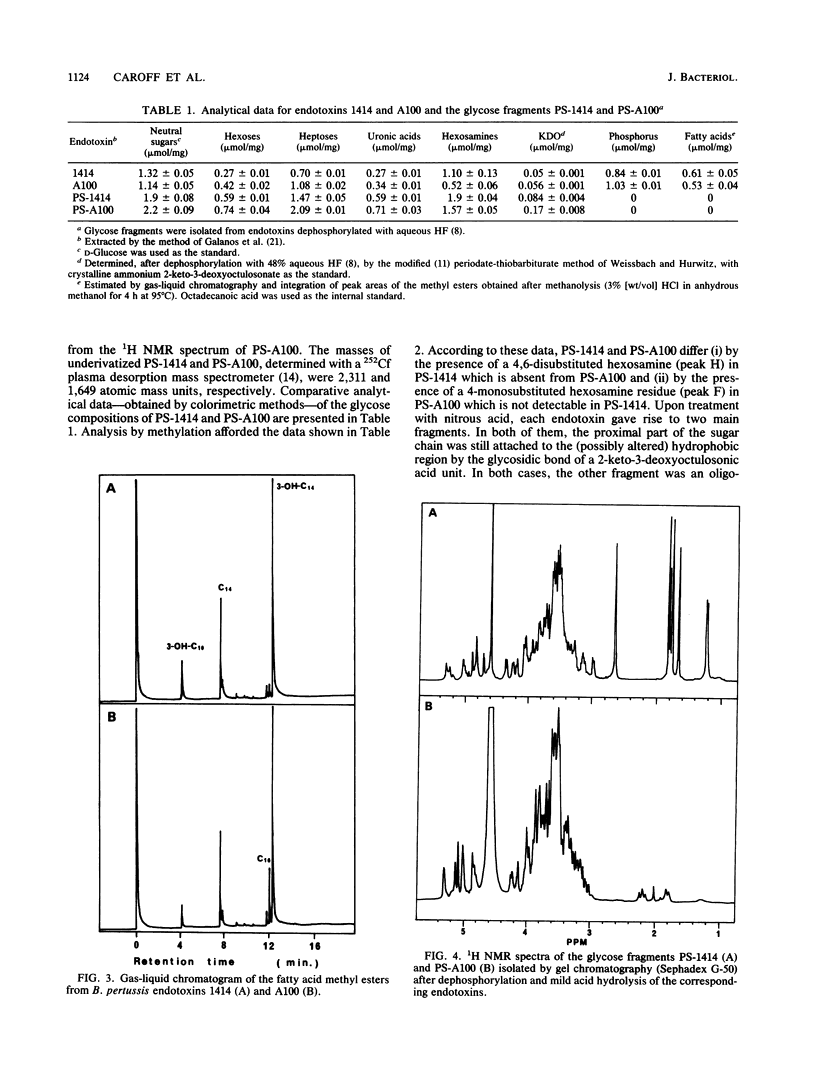
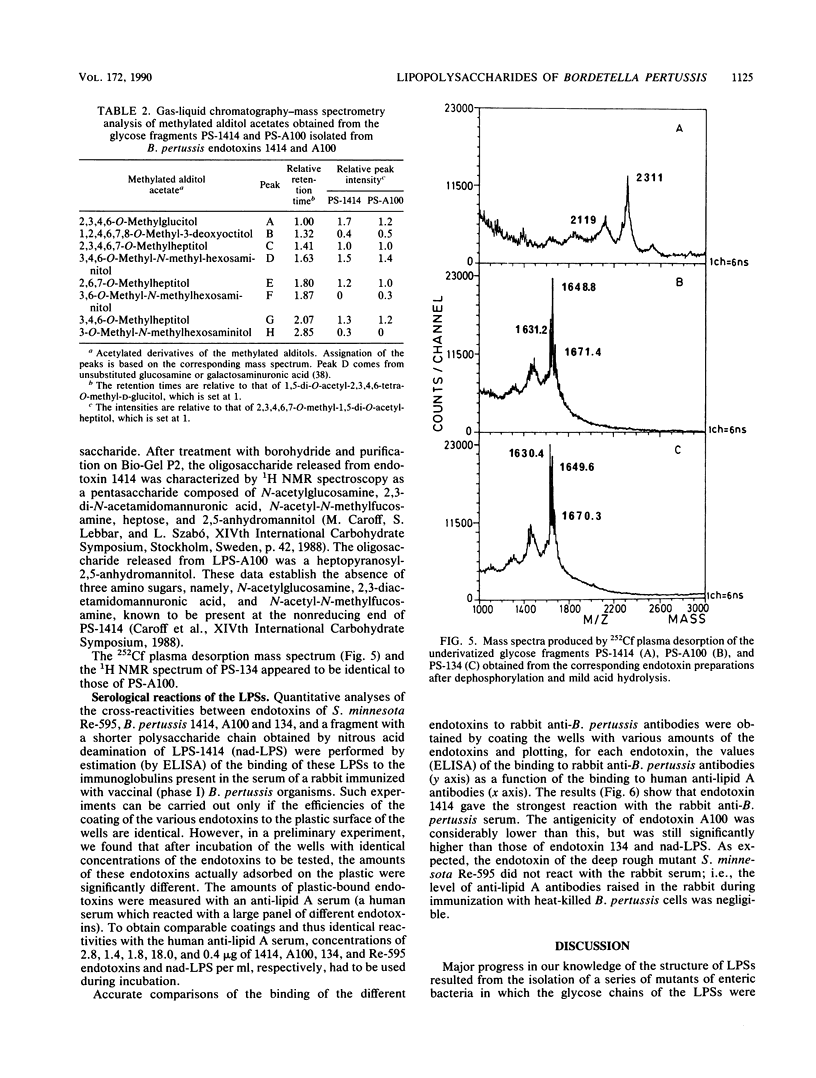
Abstract
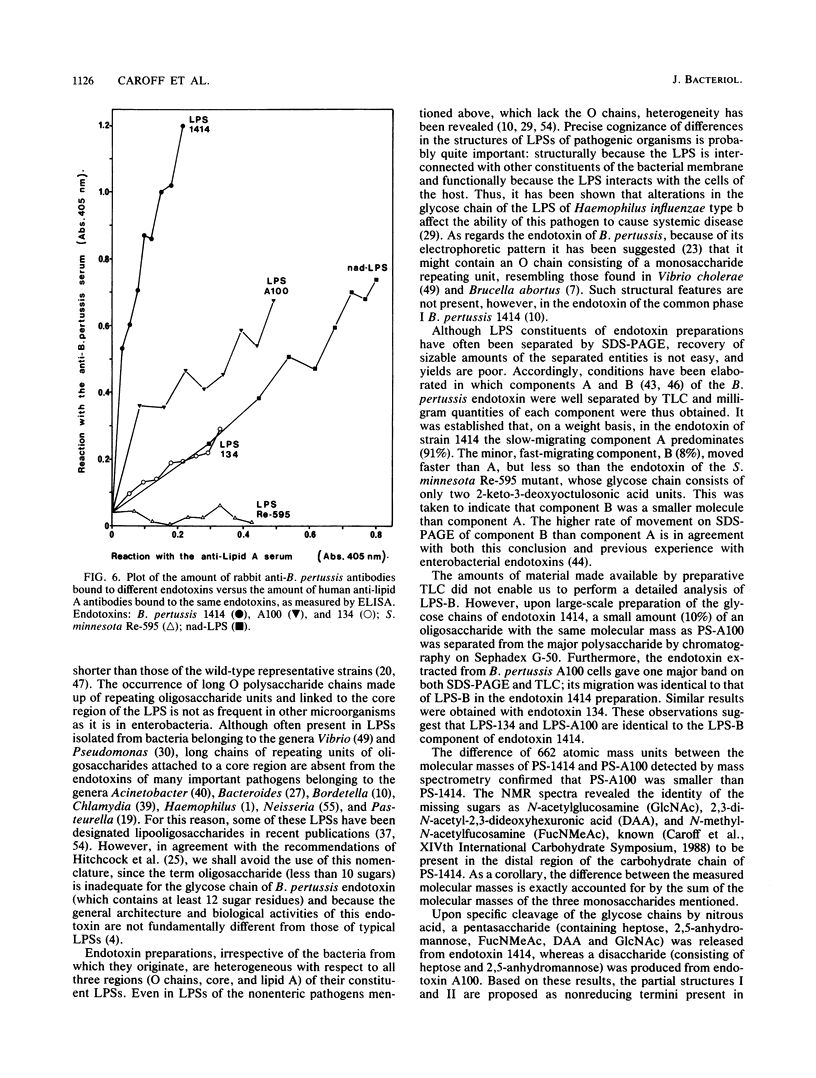
Structural and immunological differences between the two components that are usually present in unequal quantities in Bordetella pertussis endotoxin preparations and are visualized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis have been studied by using strains 1414, A100, and 134, all in phase I. According to analyses by both sodium dodecyl sulfate-polyacrylamide gel electrophoresis and thin-layer chromatography, the minor (8%) component of the endotoxin of strain 1414 (endotoxin 1414) appeared to be the predominating component of endotoxins A100 and 134. The masses of the carbohydrate chains isolated from endotoxin A100 and from the major component of endotoxin 1414 were 1,649 and 2,311 atomic mass units, respectively, as determined by 252Cf plasma desorption mass spectrometry. Comparison of the 1H nuclear magnetic resonance spectra of these chains established that four N-acetyl groups, an N-methyl group, and a 6-deoxy function, which characterize the nonreducing, distal trisaccharide of the glycose chain of strain 1414, were absent from that of strain A100. The antigenicity of endotoxin 1414, as measured by enzyme-linked immunosorbent assay, was higher than that of endotoxin A100, but fell below it when the glycose chain of endotoxin 1414 was deprived of seven sugars by treatment with nitrous acid. This observation suggests that at least three (distal, proximal, and intermediate) regions of the glycose chain of endotoxin 1414 carry antigenic determinants. One of these, located in the distal trisaccharide, is absent from both endotoxins A100 and 134.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Flesher A., Shaw S., Harding A. L., Smith D. H. Phenotypic and genetic variation in the susceptibility of Haemophilus influenzae type b to antibodies to somatic antigens. J Clin Invest. 1980 Apr;65(4):885–891. doi: 10.1172/JCI109741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprile M. A., Wardlaw A. C. Immunochemical studies on the lipopolysaccharides of Bordetella pertussis. Can J Microbiol. 1973 Feb;19(2):231–239. doi: 10.1139/m73-035. [DOI] [PubMed] [Google Scholar]
- Arai H., Munoz J. J. Fimbrial hemagglutinin in stationary and shake cultures of Bordetella pertussis. Infect Immun. 1979 Aug;25(2):764–767. doi: 10.1128/iai.25.2.764-767.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayme G., Caroff M., Chaby R., Haeffner-Cavaillon N., Le Dur A., Moreau M., Muset M., Mynard M. C., Roumiantzeff M., Schulz D. Biological activities of fragments derived from Bordetella pertussis endotoxin: isolation of a nontoxic, Shwartzman-negative lipid A possessing high adjuvant properties. Infect Immun. 1980 Mar;27(3):739–745. doi: 10.1128/iai.27.3.739-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANERJEA A., MUNOZ J. Antigens of Bordetella pertussis. II. Purification of heat-labile toxin. J Bacteriol. 1962 Aug;84:269–274. doi: 10.1128/jb.84.2.269-274.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff M., Bundle D. R., Perry M. B., Cherwonogrodzky J. W., Duncan J. R. Antigenic S-type lipopolysaccharide of Brucella abortus 1119-3. Infect Immun. 1984 Nov;46(2):384–388. doi: 10.1128/iai.46.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff M., Tacken A., Szabó L. Detergent-accelerated hydrolysis of bacterial endotoxins and determination of the anomeric configuration of the glycosyl phosphate present in the "isolated lipid A" fragment of the Bordetella pertussis endotoxin. Carbohydr Res. 1988 Apr 15;175(2):273–282. doi: 10.1016/0008-6215(88)84149-1. [DOI] [PubMed] [Google Scholar]
- Chaby R., Szabó L. 3-Deoxy-2-octulosonic acid 5-phosphate: a component of the endotoxin of Bordetella pertussis. Eur J Biochem. 1975 Nov 1;59(1):277–280. doi: 10.1111/j.1432-1033.1975.tb02452.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Wheeler M. W. Pertussis Vaccine Prepared with Phase-I Cultures Grown in Fluid Medium. Am J Public Health Nations Health. 1946 Apr;36(4):371–376. [PMC free article] [PubMed] [Google Scholar]
- Dische Z., Danilchenko A. Modifications of two color reactions of hexoses with cysteine and sulfuric acid. Anal Biochem. 1967 Oct;21(1):119–124. doi: 10.1016/0003-2697(67)90090-5. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Erler W., Feist H., Flossmann K. D., Jacob B., Pilarski A. Charakterisierung von Strukturelementen in den Lipopolysacchariden von Pasteurella multocida. Z Allg Mikrobiol. 1981;21(7):507–517. doi: 10.1002/jobm.3630210704. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Goldman W. E., Klapper D. G., Baseman J. B. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect Immun. 1982 May;36(2):782–794. doi: 10.1128/iai.36.2.782-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Lindquist U., Andersson M. Production and characterization of monoclonal antibodies directed against Bordetella pertussis lipopolysaccharide. J Clin Microbiol. 1988 Feb;26(2):188–193. doi: 10.1128/jcm.26.2.188-193.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E., Wolff J. Soluble adenylate cyclase from the culture medium of Bordetella pertussis: purification and characterization. J Bacteriol. 1976 Aug;127(2):890–898. doi: 10.1128/jb.127.2.890-898.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Leive L., Mäkelä P. H., Rietschel E. T., Strittmatter W., Morrison D. C. Lipopolysaccharide nomenclature--past, present, and future. J Bacteriol. 1986 Jun;166(3):699–705. doi: 10.1128/jb.166.3.699-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J. I., Jr, Wunderlich A. C., Lyons J., Davis C. E., Guiney D. G., Braude A. I. Role of magnesium in the enzyme-linked immunosorbent assay for lipopolysaccharides of rough Escherichia coli strain J5 and Neisseria gonorrhoeae. J Infect Dis. 1980 Oct;142(4):532–537. doi: 10.1093/infdis/142.4.532. [DOI] [PubMed] [Google Scholar]
- KASUGA T., NAKASE Y., UKISHIMA K., TAKATSU K. Studies on Haemophilus pertussis. I. Antigen structure of H. pertussis and its phases. Kitasato Arch Exp Med. 1953 Nov;26(2-3):121–133. [PubMed] [Google Scholar]
- Kasper D. L. Chemical and biological characterization of the lipopolysaccharide of Bacteroides fragilis subspecies fragilis. J Infect Dis. 1976 Jul;134(1):59–66. doi: 10.1093/infdis/134.1.59. [DOI] [PubMed] [Google Scholar]
- Kimura A., Hansen E. J. Antigenic and phenotypic variations of Haemophilus influenzae type b lipopolysaccharide and their relationship to virulence. Infect Immun. 1986 Jan;51(1):69–79. doi: 10.1128/iai.51.1.69-79.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knirel Y. A., Vinogradov E. V., Shashkov A. S., Dmitriev B. A., Kochetkov N. K., Stanislavsky E. S., Mashilova G. M. Somatic antigens of Pseudomonas aeruginosa. The structure of the O-specific polysaccharide chains of Ps.aeruginosa O:2 (Lanyi) lipopolysaccharides. Eur J Biochem. 1982 Jun 15;125(1):221–227. doi: 10.1111/j.1432-1033.1982.tb06672.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Dur A., Chaby R., Szabó L. Isolation of two protein-free and chemically different lipopolysaccharides from Bordetella pertussis phenol-extracted endotoxin. J Bacteriol. 1980 Jul;143(1):78–88. doi: 10.1128/jb.143.1.78-88.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebbar S., Cavaillon J. M., Caroff M., Ledur A., Brade H., Sarfati R., Haeffner-Cavaillon N. Molecular requirement for interleukin 1 induction by lipopolysaccharide-stimulated human monocytes: involvement of the heptosyl-2-keto-3-deoxyoctulosonate region. Eur J Immunol. 1986 Jan;16(1):87–91. doi: 10.1002/eji.1830160117. [DOI] [PubMed] [Google Scholar]
- Li Z. M., Cowell J. L., Brennan M. J., Burns D. L., Manclark C. R. Agglutinating monoclonal antibodies that specifically recognize lipooligosaccharide A of Bordetella pertussis. Infect Immun. 1988 Mar;56(3):699–702. doi: 10.1128/iai.56.3.699-702.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg B., Lönngren J. Methylation analysis of complex carbohydrates: general procedure and application for sequence analysis. Methods Enzymol. 1978;50:3–33. doi: 10.1016/0076-6879(78)50003-7. [DOI] [PubMed] [Google Scholar]
- MACLENNAN A. P. Specific lipopolysaccharides of Bordetella. Biochem J. 1960 Feb;74:398–409. doi: 10.1042/bj0740398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M., Chaby R., Szabo L. Isolation of a trisaccharide containing 2-amino-2-deoxy-D-galacturonic acid from the Bordetella pertussis endotoxin. J Bacteriol. 1982 Apr;150(1):27–35. doi: 10.1128/jb.150.1.27-35.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen M., Leinonen M., Saikku P., Mäkelä P. H. The genus-specific antigen of Chlamydia: resemblance to the lipopolysaccharide of enteric bacteria. Science. 1983 Jun 17;220(4603):1279–1281. doi: 10.1126/science.6344216. [DOI] [PubMed] [Google Scholar]
- Nurminen M., Wahlström E., Kleemola M., Leinonen M., Saikku P., Mäkelä P. H. Immunologically related ketodeoxyoctonate-containing structures in Chlamydia trachomatis, Re mutants of Salmonella species, and Acinetobacter calcoaceticus var. anitratus. Infect Immun. 1984 Jun;44(3):609–613. doi: 10.1128/iai.44.3.609-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Parker C. D., Branes L. V., Armstrong S. K., Frank D. W., Cole A. Cell surface antigens of Bordetella pertussis. Dev Biol Stand. 1985;61:123–136. [PubMed] [Google Scholar]
- Peppler M. S. Isolation and characterization of isogenic pairs of domed hemolytic and flat nonhemolytic colony types of Bordetella pertussis. Infect Immun. 1982 Mar;35(3):840–851. doi: 10.1128/iai.35.3.840-851.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppler M. S. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun. 1984 Jan;43(1):224–232. doi: 10.1128/iai.43.1.224-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P., Stirm S., Jann B., Jann K., Boman H. G. Cell-wall lipopolysaccharides of ampicillin-resistant mutants of Escherichia coli K-12. Eur J Biochem. 1976 Jul 1;66(2):369–377. doi: 10.1111/j.1432-1033.1976.tb10526.x. [DOI] [PubMed] [Google Scholar]
- Preston N. W., Surapatana N., Carter E. J. A reappraisal of serotype factors 4, 5 and 6 of Bordetella pertussis. J Hyg (Lond) 1982 Feb;88(1):39–46. doi: 10.1017/s0022172400069874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond J. W. The structure of the O-antigenic side chain of the lipopolysaccharide of Vibrio cholerae 569B (Inaba). Biochim Biophys Acta. 1979 May 1;584(2):346–352. doi: 10.1016/0304-4165(79)90280-0. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Bray D., Dankert B. M., Wright A. Direction of chain growth in polysaccharide synthesis. Science. 1967 Dec 22;158(3808):1536–1542. doi: 10.1126/science.158.3808.1536. [DOI] [PubMed] [Google Scholar]
- Sato Y., Cowell J. L., Sato H., Burstyn D. G., Manclark C. R. Separation and purification of the hemagglutinins from Bordetella pertussis. Infect Immun. 1983 Jul;41(1):313–320. doi: 10.1128/iai.41.1.313-320.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Boykins R., Frasch C. E. Heterogeneity and variation among Neisseria meningitidis lipopolysaccharides. J Bacteriol. 1983 Aug;155(2):498–504. doi: 10.1128/jb.155.2.498-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Yuasa R., Nakane K., Nikaido H. Structure of cell wall lipopolysaccharide from Salmonella typhimurium. Structure of lipopolysaccharide from a Semirough mutant. Eur J Biochem. 1970 Jul;15(1):63–71. doi: 10.1111/j.1432-1033.1970.tb00976.x. [DOI] [PubMed] [Google Scholar]