Abstract
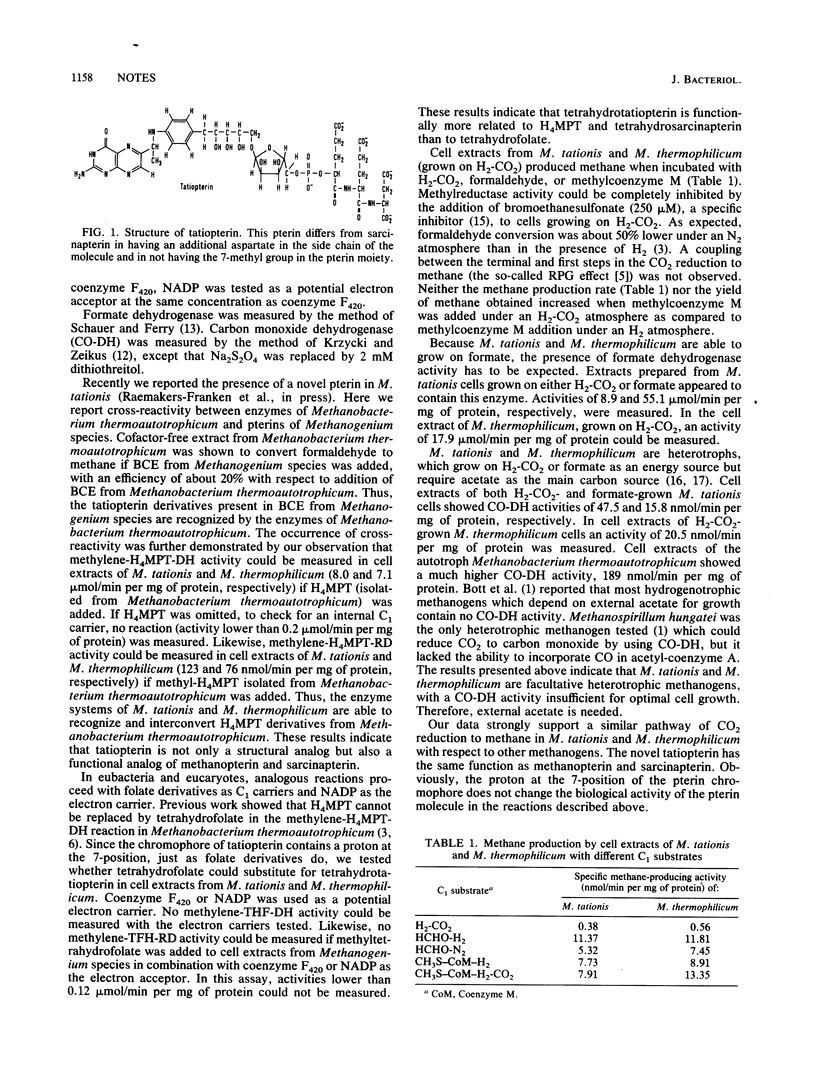
The pathway of CO2 reduction to methane in Methanogenium tationis and Methanogenium thermophilicum is similar to that observed in other methanogens. In M. tationis a novel pterin, tatiopterin, is present. This pterin appears to be a structural and functional analog of methanopterin and sarcinapterin. Folate could not substitute for tatiopterin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Escalante-Semerena J. C., Rinehart K. L., Jr, Wolfe R. S. Tetrahydromethanopterin, a carbon carrier in methanogenesis. J Biol Chem. 1984 Aug 10;259(15):9447–9455. [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Wolfe R. S. Formaldehyde oxidation and methanogenesis. J Bacteriol. 1984 May;158(2):721–726. doi: 10.1128/jb.158.2.721-726.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorris L. G., van der Drift C., Vogels G. D. 7-methylpterin derivatives in extracts of methanogens characterized by a relatively low methanopterin content. Biofactors. 1988 Jan;1(1):105–109. [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Stimulation of CO2 reduction to methane by methylcoenzyme M in extracts Methanobacterium. Biochem Biophys Res Commun. 1977 Jun 6;76(3):790–795. doi: 10.1016/0006-291x(77)91570-4. [DOI] [PubMed] [Google Scholar]
- Hartzell P. L., Zvilius G., Escalante-Semerena J. C., Donnelly M. I. Coenzyme F420 dependence of the methylenetetrahydromethanopterin dehydrogenase of Methanobacterium thermoautotrophicum. Biochem Biophys Res Commun. 1985 Dec 31;133(3):884–890. doi: 10.1016/0006-291x(85)91218-5. [DOI] [PubMed] [Google Scholar]
- Hutten T. J., De Jong M. H., Peeters B. P., van der Drift C., Vogels G. D. Coenzyme M derivatives and their effects on methane formation from carbon dioxide and methanol by cell extracts of Methanosarcina barkeri. J Bacteriol. 1981 Jan;145(1):27–34. doi: 10.1128/jb.145.1.27-34.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. J., Donnelly M. I., Wolfe R. S. Evidence of a common pathway of carbon dioxide reduction to methane in methanogens. J Bacteriol. 1985 Jul;163(1):126–131. doi: 10.1128/jb.163.1.126-131.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltjens J. T., Kraft H. J., Damen W. G., van der Drift C., Vogels G. D. Stimulation of the methylcoenzyme M reduction by uridine-5'-diphospho-sugars in cell-free extracts of Methanobacterium thermoautotrophicum (strain delta H). Eur J Biochem. 1989 Sep 15;184(2):395–403. doi: 10.1111/j.1432-1033.1989.tb15031.x. [DOI] [PubMed] [Google Scholar]
- Keltjens J. T., van Erp R., Mooijaart R. J., van der Drift C., Vogels G. D. Inorganic pyrophosphate synthesis during methanogenesis from methylcoenzyme M by cell-free extracts of Methanobacterium thermoautotrophicum (strain delta H). Eur J Biochem. 1988 Mar 1;172(2):471–476. doi: 10.1111/j.1432-1033.1988.tb13912.x. [DOI] [PubMed] [Google Scholar]
- Krzycki J. A., Zeikus J. G. Characterization and purification of carbon monoxide dehydrogenase from Methanosarcina barkeri. J Bacteriol. 1984 Apr;158(1):231–237. doi: 10.1128/jb.158.1.231-237.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Properties of formate dehydrogenase in Methanobacterium formicicum. J Bacteriol. 1982 Apr;150(1):1–7. doi: 10.1128/jb.150.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


