Abstract
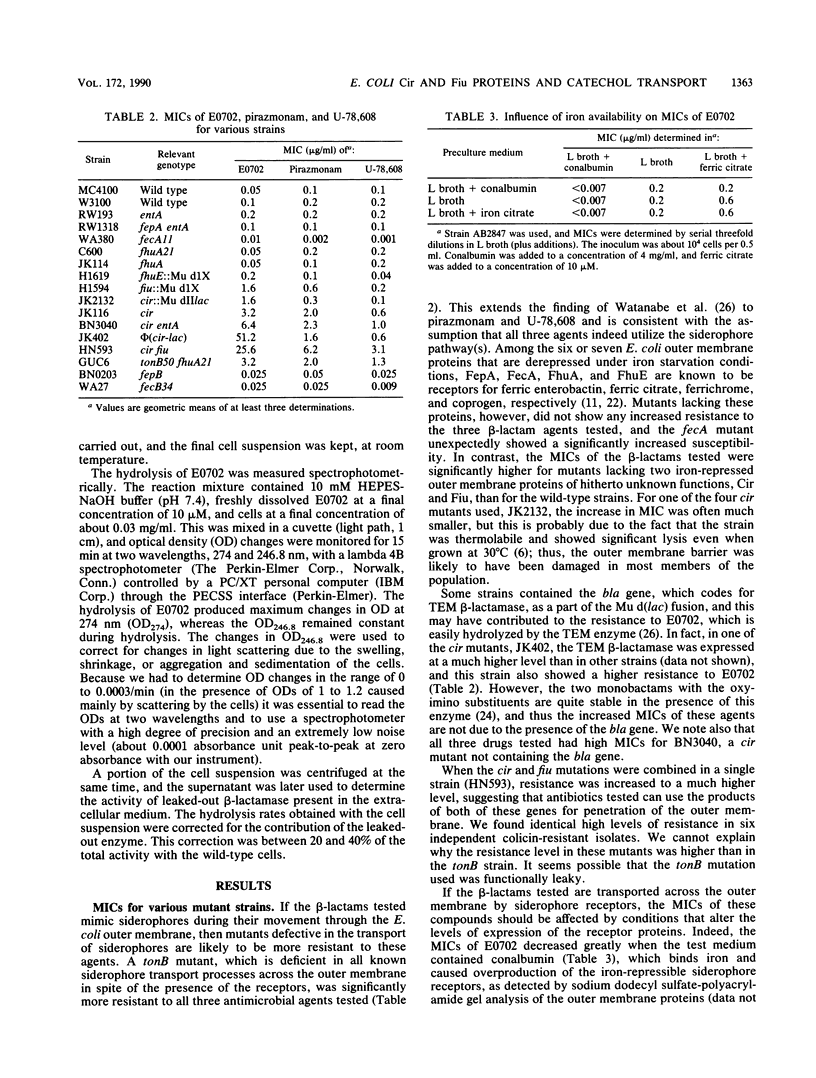
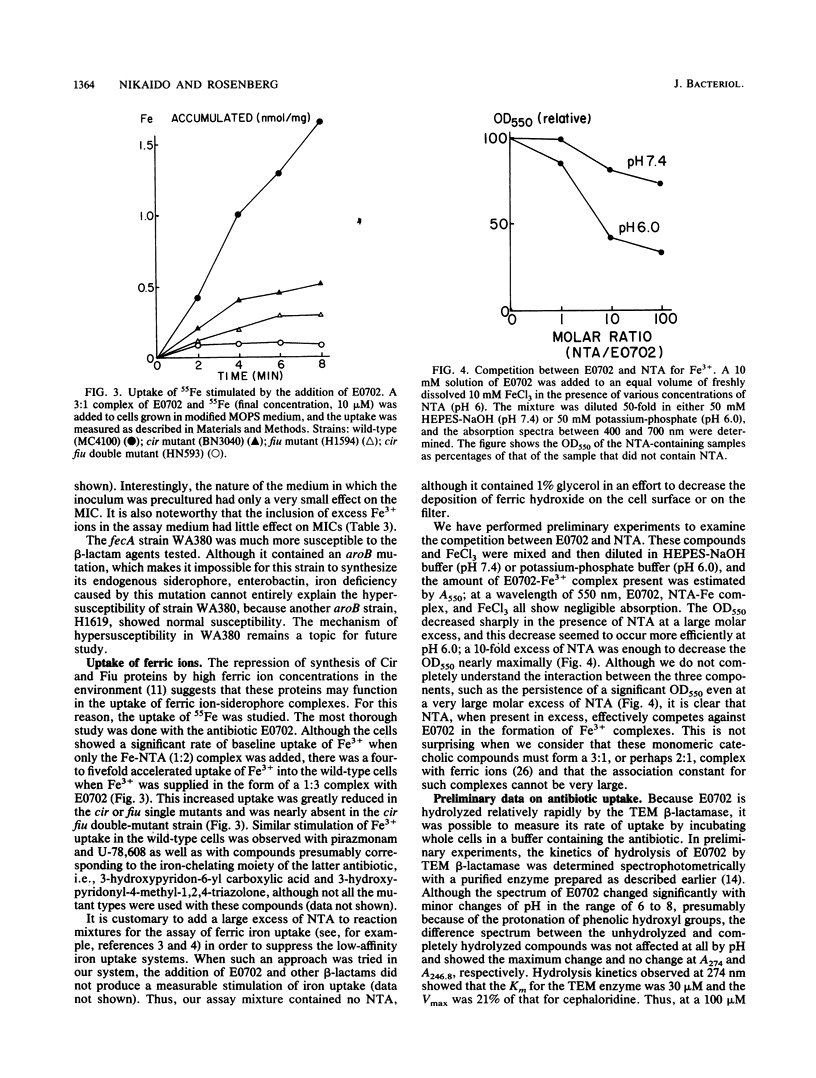
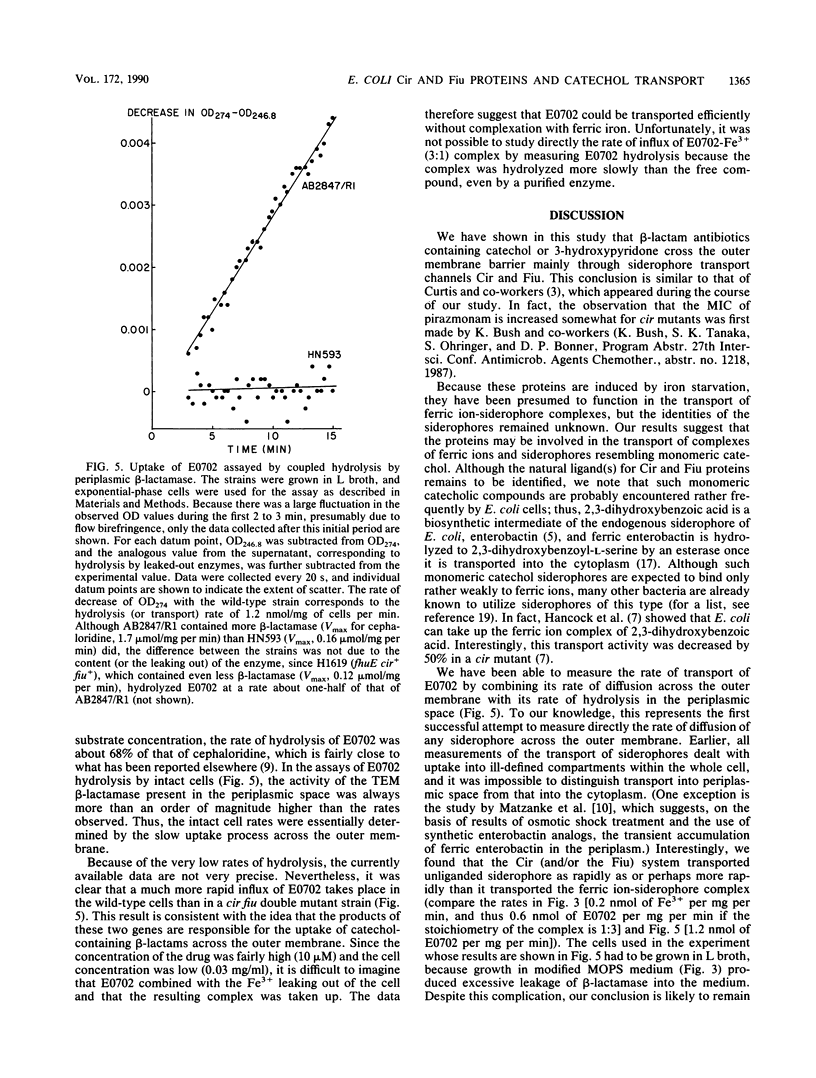
Recently, beta-lactam agents containing iron-chelating moieties, such as E0702, which contains catechol, and pirazmonam and U-78,608, which contain 3-hydroxypyridone, have been developed. By determining the susceptibility to these agents of Escherichia coli mutants lacking various iron-repressible outer membrane proteins, we showed that two of these proteins with hitherto unknown functions, Fiu and Cir, were apparently involved in the transport of monomeric catechol and its analogs. These results confirm the conclusion of Curtis and co-workers, which was obtained by using a different set of catechol-containing antibiotics (N. A. C. Curtis, R. L. Eisenstadt, S. J. East, R. J. Cornford, L. A. Walker, and A. J. White, Antimicrob. Agents Chemother. 32:1879-1886, 1988). E0702 was shown to enhance the uptake of radioactive ferric iron into wild-type cells but not into cir fiu double mutants. By combining the influx of E0702 with its hydrolysis by a periplasmic beta-lactamase, we showed that the wild-type cells transported unliganded E0702 at a rate comparable to or even higher than the rate of transport of the E0702-Fe3+ complex. We postulate that the main function of Cir and Fiu may be to recapture the hydrolytic products of enterobactin, such as 2,3-dihydroxybenzoic acid and 2,3-dihydroxybenzoylserine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Ames G. F., Young J. D., Tsuchiya D., Lecocq J. Illicit transport: the oligopeptide permease. Proc Natl Acad Sci U S A. 1973 Feb;70(2):456–458. doi: 10.1073/pnas.70.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardelli J., Konisky J. Isolation and characterization of an Escherichia coli mutant tolerant to colicins Ia and Ib. J Bacteriol. 1974 Aug;119(2):379–385. doi: 10.1128/jb.119.2.379-385.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Eisenstadt R. L., East S. J., Cornford R. J., Walker L. A., White A. J. Iron-regulated outer membrane proteins of Escherichia coli K-12 and mechanism of action of catechol-substituted cephalosporins. Antimicrob Agents Chemother. 1988 Dec;32(12):1879–1886. doi: 10.1128/aac.32.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker D. J., Matzanke B. F., Raymond K. N. Recognition and transport of ferric enterobactin in Escherichia coli. J Bacteriol. 1986 Aug;167(2):666–673. doi: 10.1128/jb.167.2.666-673.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Pittard J. Pathways of biosynthesis of aromatic amino acids and vitamins and their control in microorganisms. Bacteriol Rev. 1968 Dec;32(4 Pt 2):465–492. [PMC free article] [PubMed] [Google Scholar]
- Griggs D. W., Tharp B. B., Konisky J. Cloning and promoter identification of the iron-regulated cir gene of Escherichia coli. J Bacteriol. 1987 Dec;169(12):5343–5352. doi: 10.1128/jb.169.12.5343-5352.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Hantke K., Braun V. Iron transport in Escherichia coli K-12. 2,3-Dihydroxybenzoate-promoted iron uptake. Arch Microbiol. 1977 Sep 28;114(3):231–239. doi: 10.1007/BF00446867. [DOI] [PubMed] [Google Scholar]
- Hollifield W. C., Jr, Fiss E. H., Neilands J. B. Modification of a ferric enterobactin receptor protein from the outer membrane of Escherichia coli. Biochem Biophys Res Commun. 1978 Jul 28;83(2):739–746. doi: 10.1016/0006-291x(78)91051-3. [DOI] [PubMed] [Google Scholar]
- Katsu K., Kitoh K., Inoue M., Mitsuhashi S. In vitro antibacterial activity of E-0702, a new semisynthetic cephalosporin. Antimicrob Agents Chemother. 1982 Aug;22(2):181–185. doi: 10.1128/aac.22.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzanke B. F., Ecker D. J., Yang T. S., Huynh B. H., Müller G., Raymond K. N. Escherichia coli iron enterobactin uptake monitored by Mössbauer spectroscopy. J Bacteriol. 1986 Aug;167(2):674–680. doi: 10.1128/jb.167.2.674-680.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Normark S. Sensitivity of Escherichia coli to various beta-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic beta-lactamases: a quantitative predictive treatment. Mol Microbiol. 1987 Jul;1(1):29–36. doi: 10.1111/j.1365-2958.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien I. G., Cox G. B., Gibson F. Enterochelin hydrolysis and iron metabolism in Escherichia coli. Biochim Biophys Acta. 1971 Jun 22;237(3):537–549. doi: 10.1016/0304-4165(71)90274-1. [DOI] [PubMed] [Google Scholar]
- Ohi N., Aoki B., Shinozaki T., Moro K., Noto T., Nehashi T., Okazaki H., Matsunaga I. Semisynthetic beta-lactam antibiotics. I. Synthesis and antibacterial activity of new ureidopenicillin derivatives having catechol moieties. J Antibiot (Tokyo) 1986 Feb;39(2):230–241. doi: 10.7164/antibiotics.39.230. [DOI] [PubMed] [Google Scholar]
- Persmark M., Expert D., Neilands J. B. Isolation, characterization, and synthesis of chrysobactin, a compound with siderophore activity from Erwinia chrysanthemi. J Biol Chem. 1989 Feb 25;264(6):3187–3193. [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M., Hantke K., Braun V. Ferric-coprogen receptor FhuE of Escherichia coli: processing and sequence common to all TonB-dependent outer membrane receptor proteins. J Bacteriol. 1987 May;169(5):2044–2049. doi: 10.1128/jb.169.5.2044-2049.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Koster W. H., Bonner D. P. The new monobactams: chemistry and biology. J Clin Pharmacol. 1988 Feb;28(2):113–119. doi: 10.1002/j.1552-4604.1988.tb05734.x. [DOI] [PubMed] [Google Scholar]
- Wagegg W., Braun V. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein fecA. J Bacteriol. 1981 Jan;145(1):156–163. doi: 10.1128/jb.145.1.156-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N. A., Nagasu T., Katsu K., Kitoh K. E-0702, a new cephalosporin, is incorporated into Escherichia coli cells via the tonB-dependent iron transport system. Antimicrob Agents Chemother. 1987 Apr;31(4):497–504. doi: 10.1128/aac.31.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne R., Frick K., Neilands J. B. Siderophore protection against colicins M, B, V, and Ia in Escherichia coli. J Bacteriol. 1976 Apr;126(1):7–12. doi: 10.1128/jb.126.1.7-12.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsham P. L., Konisky J. Use of cir-lac operon fusions to study transcriptional regulation of the colicin Ia receptor in Escherichia coli K-12. J Bacteriol. 1981 Jan;145(1):647–650. doi: 10.1128/jb.145.1.647-650.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



