Abstract
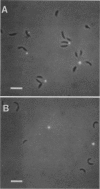
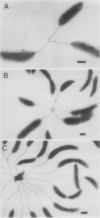
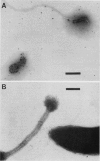
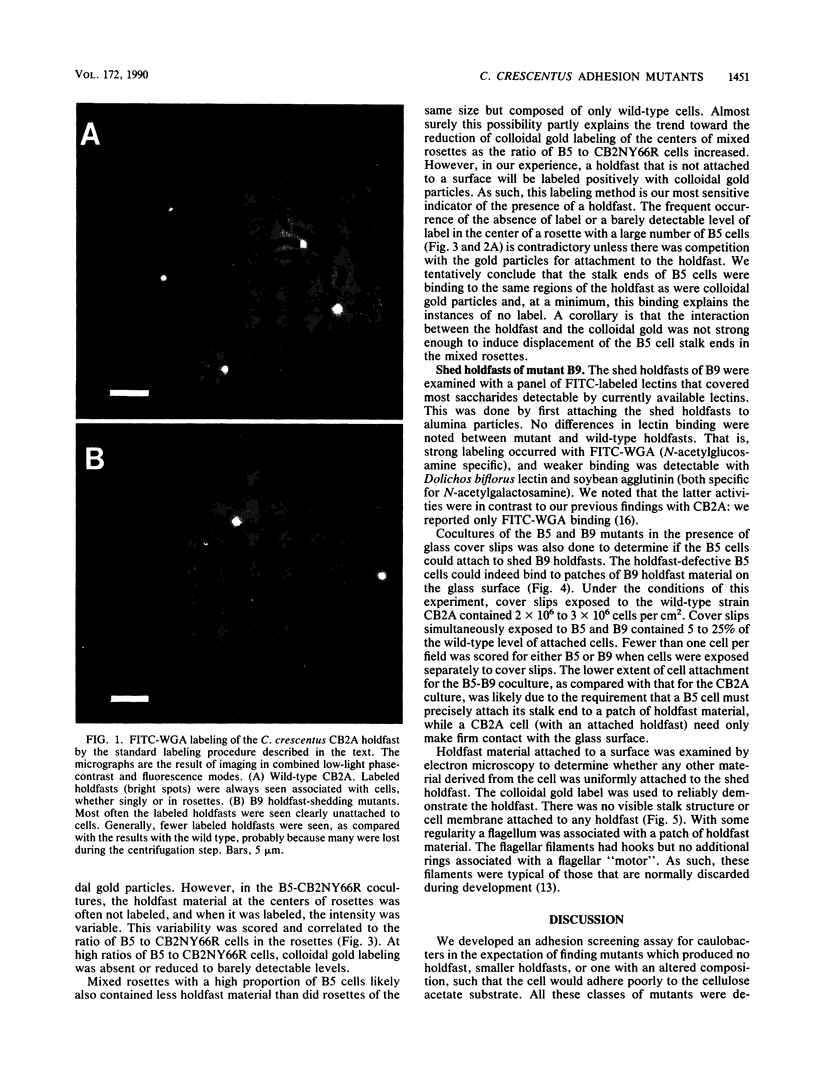
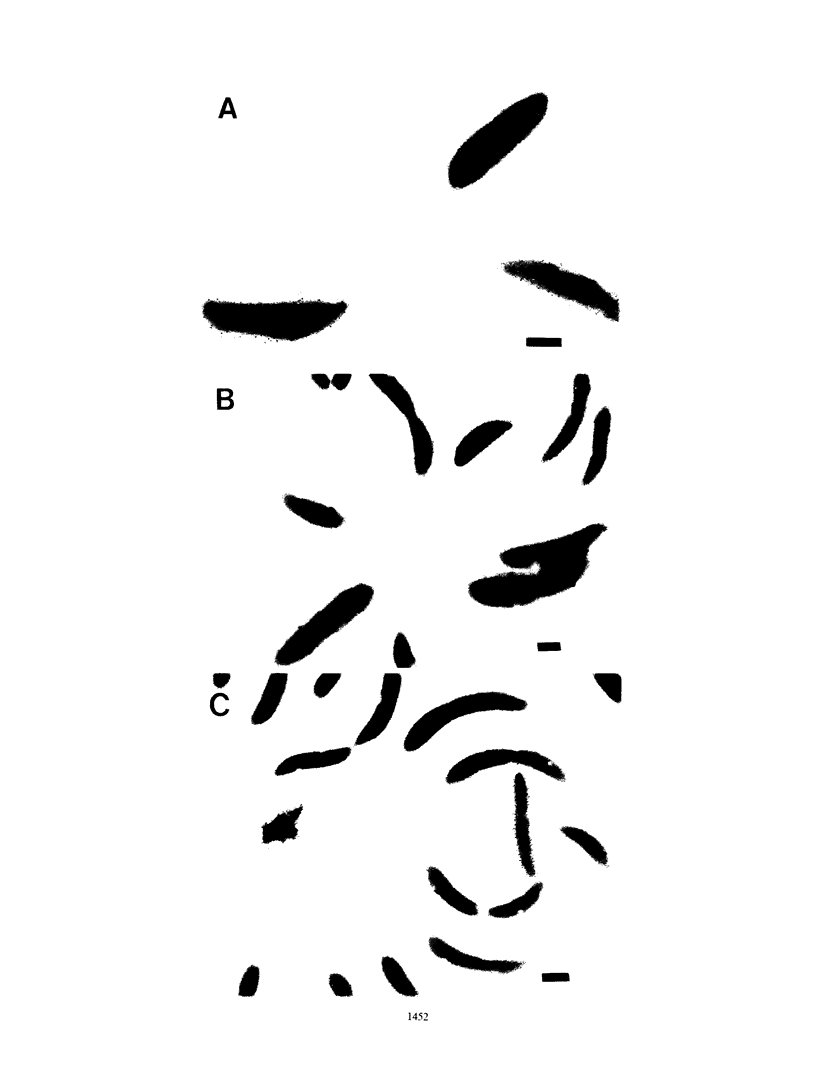
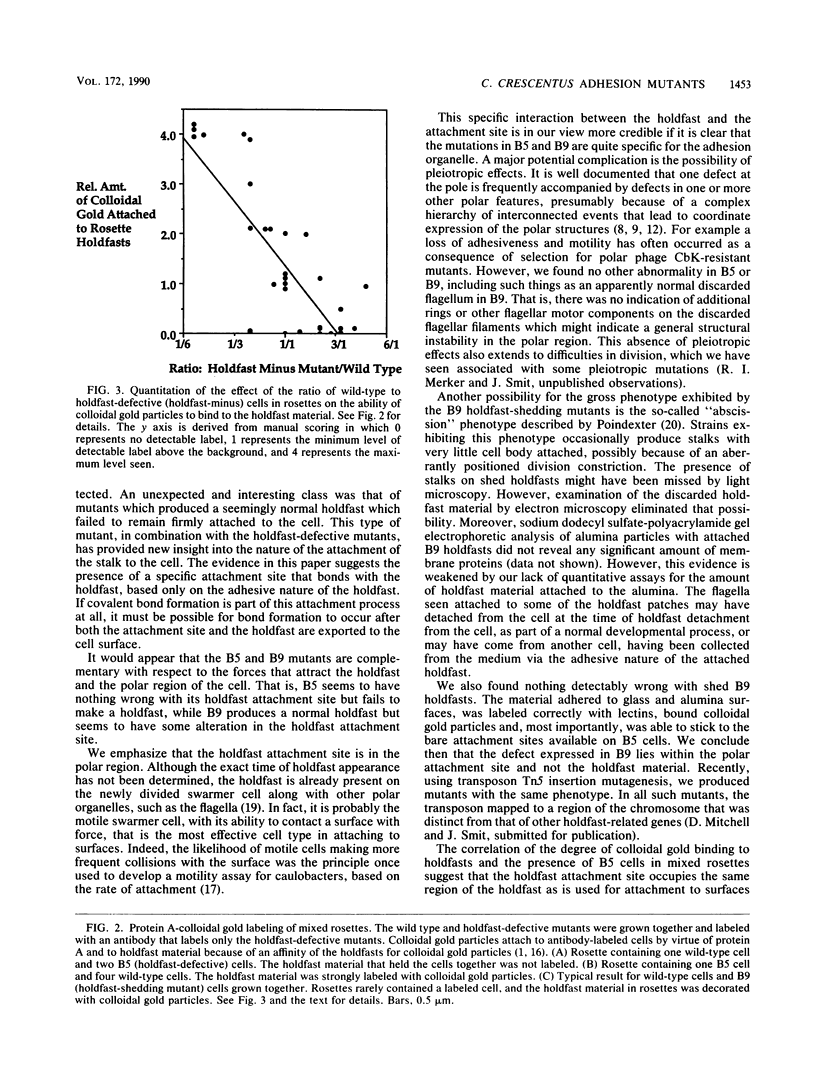
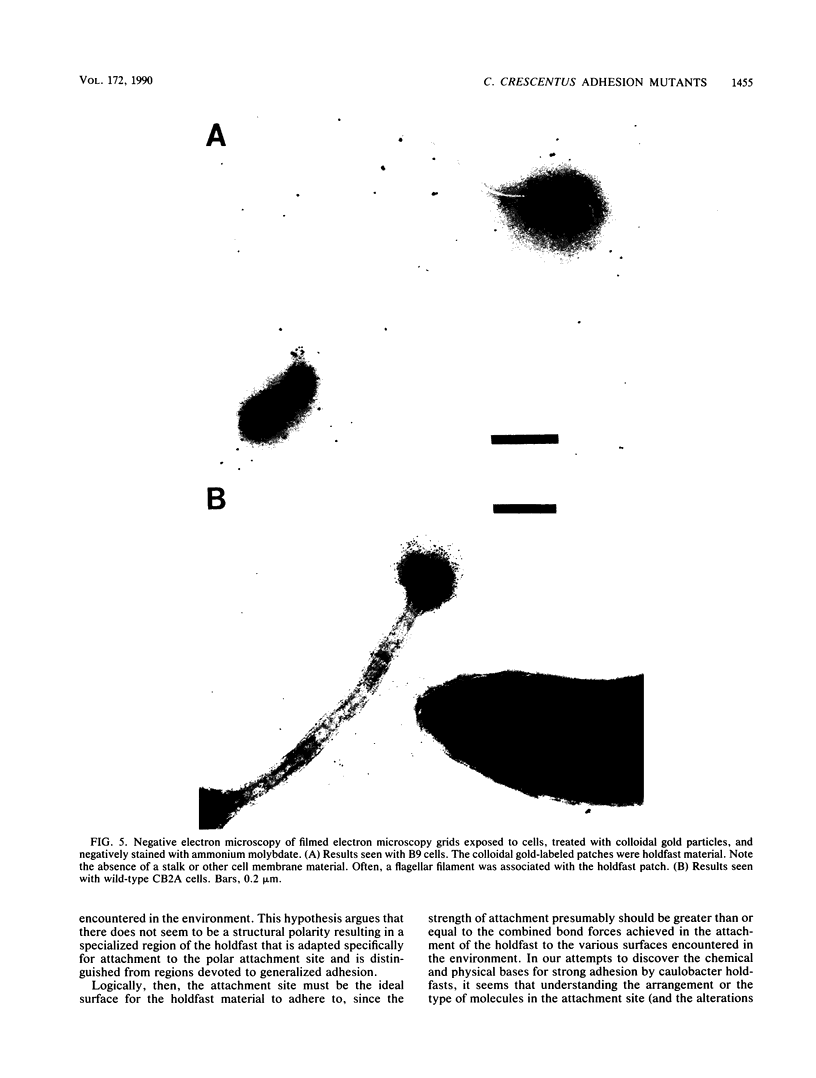
Caulobacters attach to surfaces in the environment via their holdfasts, attachment organelles located at the base of the flagellum in swarmer cells and later at the end of the cellular stalk in the stalked cells which develop from the swarmer cells. There seems to be little specificity with respect to the types of surfaces to which holdfasts adhere. A notable exception is that the holdfast of one cell does not adhere to the cell surface of another caulobacter, except by joining holdfasts, typically forming "rosettes" of stalked cells. Thus, the localized adhesion of the holdfasts to the cells is in some way a specialized attachment. We investigated this holdfast-cell attachment by developing an adhesion screening assay and analyzing several mutants of Caulobacter crescentus CB2A selected to be defective in adhesion. One class of mutants made a normal holdfast by all available criteria, yet the attachment to the cell was very weak, such that the holdfast was readily shed. Another class of mutants made no holdfast at all, but when mixed with a wild-type strain, a mutant of this class participated in rosette formation. The mutant could also attach to the discarded holdfast produced by a shedding mutant. In addition, when rosettes composed of holdfast-defective and wild-type cells were examined, an increase in the number of holdfast-defective cells was correlated with a decrease in the ability of the holdfast material at the center of the rosette to bind colloidal gold particles. Gold particles are one type of surface to which holdfasts adhere well, suggesting that the stalk end and the colloidal gold particles occupy the same sites on the holdfast substance. Taken together, the data support the interpretation that there is a specialized attachment site for the holdfast at the base of the flagellum which later becomes the end of the stalk, but not a specialized region of the holdfast for attachment to this site. Also, attachment to the cell is accomplished by bond formations that occur not only at the time of holdfast production. Thus, we propose that the attachment of the holdfast to the cell is a true adhesion process and that the stalk tip and base of the flagellum must have compositions distinctly different from that of the remainder of the caulobacter cell surface.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian-Keshishian N., Shapiro L. Stalked bacteria: properties of deoxriybonucleic acid bacteriophage phiCbK. J Virol. 1970 Jun;5(6):795–800. doi: 10.1128/jvi.5.6.795-800.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anast Nick, Smit John. Isolation and Characterization of Marine Caulobacters and Assessment of Their Potential for Genetic Experimentation. Appl Environ Microbiol. 1988 Mar;54(3):809–817. doi: 10.1128/aem.54.3.809-817.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R., Simon M., Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986 Jul;167(1):210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad R., Phelps T. J., Zeikus J. G. Gas metabolism evidence in support of the juxtaposition of hydrogen-producing and methanogenic bacteria in sewage sludge and lake sediments. Appl Environ Microbiol. 1985 Sep;50(3):595–601. doi: 10.1128/aem.50.3.595-601.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Cheng K. J., Geesey G. G., Ladd T. I., Nickel J. C., Dasgupta M., Marrie T. J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Daniels L., Belay N., Rajagopal B. S., Weimer P. J. Bacterial Methanogenesis and Growth from CO2 with Elemental Iron as the Sole Source of Electrons. Science. 1987 Jul 31;237(4814):509–511. doi: 10.1126/science.237.4814.509. [DOI] [PubMed] [Google Scholar]
- Fukuda A., Asada M., Koyasu S., Yoshida H., Yaginuma K., Okada Y. Regulation of polar morphogenesis in Caulobacter crescentus. J Bacteriol. 1981 Jan;145(1):559–572. doi: 10.1128/jb.145.1.559-572.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A., Miyakawa K., Iida H., Okada Y. Regulation of polar surface structures in Caulobacter crescentus: pleiotropic mutations affect the coordinate morphogenesis of flagella, pili and phage receptors. Mol Gen Genet. 1976 Dec 8;149(2):167–173. doi: 10.1007/BF00332885. [DOI] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Gregory D. W., Pirie B. J. Wetting agents for biological electron microscopy. I. General considerations and negative staining. J Microsc. 1973 Dec;99(3):251–255. doi: 10.1111/j.1365-2818.1973.tb04625.x. [DOI] [PubMed] [Google Scholar]
- Kurn N., Ammer S., Shapiro L. A pleiotropic mutation affecting expression of polar development events in Caulobacter crescentus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3157–3161. doi: 10.1073/pnas.71.8.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Caulobacter flagellar organelle: synthesis, compartmentation, and assembly. J Bacteriol. 1978 Sep;135(3):1062–1069. doi: 10.1128/jb.135.3.1062-1069.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker R. I., Smit J. Characterization of the adhesive holdfast of marine and freshwater caulobacters. Appl Environ Microbiol. 1988 Aug;54(8):2078–2085. doi: 10.1128/aem.54.8.2078-2085.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A. Role of transcription in the temporal control of development in Caulobacter crescentus (stalk-rifampin-RNA synthesis-DNA synthesis-motility). Proc Natl Acad Sci U S A. 1972 Feb;69(2):447–451. doi: 10.1073/pnas.69.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Jeffrey W. H. Evidence for Separate Adhesion Mechanisms for Hydrophilic and Hydrophobic Surfaces in Vibrio proteolytica. Appl Environ Microbiol. 1985 Aug;50(2):431–437. doi: 10.1128/aem.50.2.431-437.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter J. S. Selection for nonbuoyant morphological mutants of Caulobacter crescentus. J Bacteriol. 1978 Sep;135(3):1141–1145. doi: 10.1128/jb.135.3.1141-1145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. M. Observations on the adsorption of Caulobacter bacteriophages containing ribonucleic acid. J Gen Microbiol. 1966 Nov;45(2):347–353. doi: 10.1099/00221287-45-2-347. [DOI] [PubMed] [Google Scholar]
- Smit J., Agabian N. Caulobacter crescentus pili: analysis of production during development. Dev Biol. 1982 Jan;89(1):237–247. doi: 10.1016/0012-1606(82)90310-4. [DOI] [PubMed] [Google Scholar]
- Smit J., Agabian N. Cloning of the major protein of the Caulobacter crescentus periodic surface layer: detection and characterization of the cloned peptide by protein expression assays. J Bacteriol. 1984 Dec;160(3):1137–1145. doi: 10.1128/jb.160.3.1137-1145.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Grano D. A., Glaeser R. M., Agabian N. Periodic surface array in Caulobacter crescentus: fine structure and chemical analysis. J Bacteriol. 1981 Jun;146(3):1135–1150. doi: 10.1128/jb.146.3.1135-1150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Hermodson M., Agabian N. Caulobacter crescentus pilin. Purification, chemical characterization, and NH2-terminal amino acid sequence of a structural protein regulated during development. J Biol Chem. 1981 Mar 25;256(6):3092–3097. [PubMed] [Google Scholar]
- Sommer J. M., Newton A. Sequential regulation of developmental events during polar morphogenesis in Caulobacter crescentus: assembly of pili on swarmer cells requires cell separation. J Bacteriol. 1988 Jan;170(1):409–415. doi: 10.1128/jb.170.1.409-415.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Microbial exopolysaccharides -- their role in microbial adhesion in aqueous systems. Crit Rev Microbiol. 1983;10(2):173–201. doi: 10.3109/10408418209113562. [DOI] [PubMed] [Google Scholar]
- Uhlinger D. J., White D. C. Relationship Between Physiological Status and Formation of Extracellular Polysaccharide Glycocalyx in Pseudomonas atlantica. Appl Environ Microbiol. 1983 Jan;45(1):64–70. doi: 10.1128/aem.45.1.64-70.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]