Abstract
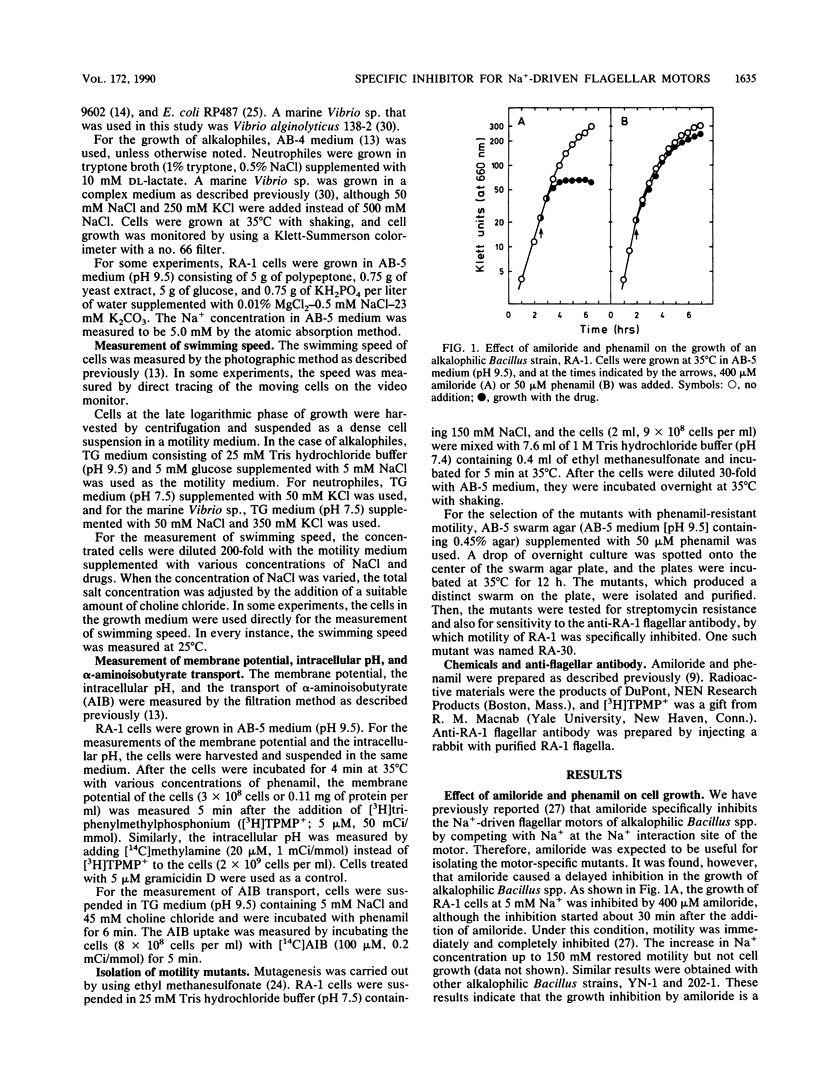
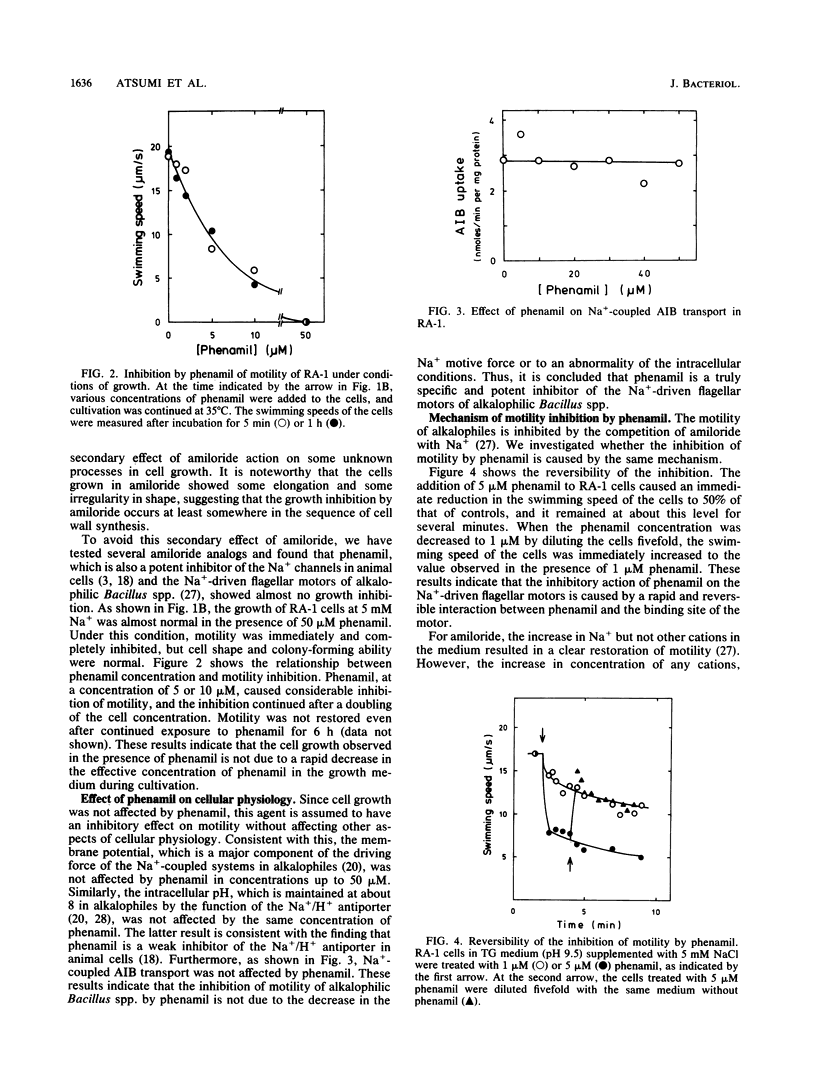
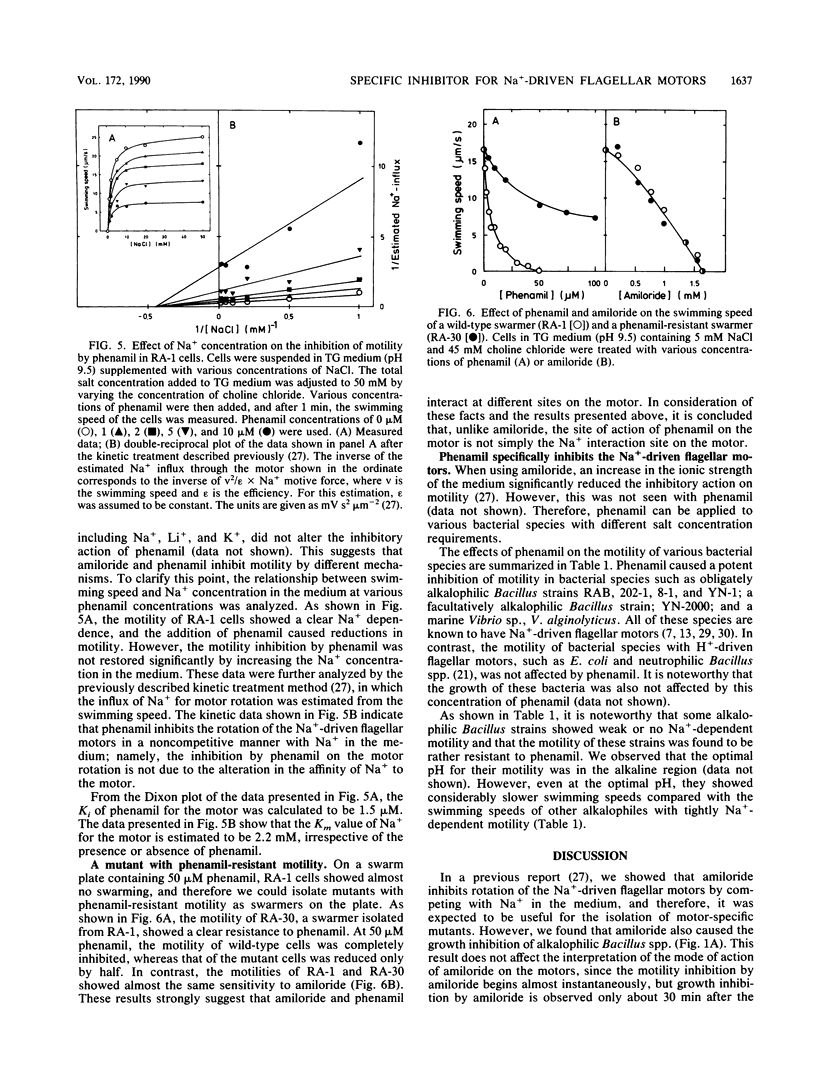
Amiloride, a specific inhibitor for the Na(+)-driven flagellar motors of alkalophilic Bacillus strains, was found to cause growth inhibition; therefore, the use of amiloride for the isolation of motility mutants was difficult. On the other hand, phenamil, an amiloride analog, inhibited motor rotation without affecting cell growth. A concentration of 50 microM phenamil completely inhibited the motility of strain RA-1 but showed no effect on the membrane potential, the intracellular pH, or Na(+)-coupled amino acid transport, which was consistent with the fact that there was no effect on cell growth. Kinetic analysis of the inhibition of motility by phenamil indicated that the inhibition was noncompetitive with Na+ in the medium. A motility mutant was isolated as a swarmer on a swarm agar plate containing 50 microM phenamil. The motility of the mutant showed an increased resistance to phenamil but normal sensitivity to amiloride. These results suggest that phenamil and amiloride interact at different sites on the motor. By examining various bacterial species, phenamil was found to be a specific and potent inhibitor for the Na(+)-driven flaggellar motors not only in various strains of alkalophilic Bacillus spp. but also in a marine Vibrio sp.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asher C., Cragoe E. J., Jr, Garty H. Effects of amiloride analogues on Na+ transport in toad bladder membrane vesicles. Evidence for two electrogenic transporters with different affinities toward pyrazinecarboxamides. J Biol Chem. 1987 Jun 25;262(18):8566–8573. [PubMed] [Google Scholar]
- Barbry P., Chassande O., Duval D., Rousseau B., Frelin C., Lazdunski M. Biochemical identification of two types of phenamil binding sites associated with amiloride-sensitive Na+ channels. Biochemistry. 1989 May 2;28(9):3744–3749. doi: 10.1021/bi00435a018. [DOI] [PubMed] [Google Scholar]
- Benos D. J. Amiloride: a molecular probe of sodium transport in tissues and cells. Am J Physiol. 1982 Mar;242(3):C131–C145. doi: 10.1152/ajpcell.1982.242.3.C131. [DOI] [PubMed] [Google Scholar]
- Blair D. F., Berg H. C. Restoration of torque in defective flagellar motors. Science. 1988 Dec 23;242(4886):1678–1681. doi: 10.1126/science.2849208. [DOI] [PubMed] [Google Scholar]
- Block S. M., Berg H. C. Successive incorporation of force-generating units in the bacterial rotary motor. 1984 May 31-Jun 6Nature. 309(5967):470–472. doi: 10.1038/309470a0. [DOI] [PubMed] [Google Scholar]
- Chun S. Y., Parkinson J. S. Bacterial motility: membrane topology of the Escherichia coli MotB protein. Science. 1988 Jan 15;239(4837):276–278. doi: 10.1126/science.2447650. [DOI] [PubMed] [Google Scholar]
- Cragoe E. J., Jr, Woltersdorf O. W., Jr, Bicking J. B., Kwong S. F., Jones J. H. Pyrazine diuretics. II. N-amidino-3-amino-5-substituted 6-halopyrazinecarboxamides. J Med Chem. 1967 Jan;10(1):66–75. doi: 10.1021/jm00313a014. [DOI] [PubMed] [Google Scholar]
- Hirota N., Imae Y. Na+-driven flagellar motors of an alkalophilic Bacillus strain YN-1. J Biol Chem. 1983 Sep 10;258(17):10577–10581. [PubMed] [Google Scholar]
- Imae Y., Atsumi T. Na+-driven bacterial flagellar motors. J Bioenerg Biomembr. 1989 Dec;21(6):705–716. doi: 10.1007/BF00762688. [DOI] [PubMed] [Google Scholar]
- Imae Y., Matsukura H., Kobayasi S. Sodium-driven flagellar motors of alkalophilic Bacillus. Methods Enzymol. 1986;125:582–592. doi: 10.1016/s0076-6879(86)25047-8. [DOI] [PubMed] [Google Scholar]
- Khan S., Dapice M., Reese T. S. Effects of mot gene expression on the structure of the flagellar motor. J Mol Biol. 1988 Aug 5;202(3):575–584. doi: 10.1016/0022-2836(88)90287-2. [DOI] [PubMed] [Google Scholar]
- Kitada M., Horikoshi K. Bioenergetic properties of alkalophilic Bacillus sp. strain C-59 on an alkaline medium containing K2CO3. J Bacteriol. 1987 Dec;169(12):5761–5765. doi: 10.1128/jb.169.12.5761-5765.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M., Onda K., Horikoshi K. The sodium/proton antiport system in a newly isolated alkalophilic Bacillus sp. J Bacteriol. 1989 Apr;171(4):1879–1884. doi: 10.1128/jb.171.4.1879-1884.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyman T. R., Cragoe E. J., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988 Oct;105(1):1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Guffanti A. A., Bornstein R. F., Hoffstein J. A sodium requirement for growth, solute transport, and pH homeostasis in Bacillus firmus RAB. J Biol Chem. 1982 Feb 25;257(4):1885–1889. [PubMed] [Google Scholar]
- Krulwich T. A., Hicks D. B., Seto-Young D., Guffanti A. A. The bioenergetics of alkalophilic bacilli. Crit Rev Microbiol. 1988;16(1):15–36. doi: 10.3109/10408418809104466. [DOI] [PubMed] [Google Scholar]
- Manson M. D., Tedesco P., Berg H. C., Harold F. M., Van der Drift C. A protonmotive force drives bacterial flagella. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsura S., Shioi J., Imae Y. Motility in Bacillus subtilis driven by an artificial protonmotive force. FEBS Lett. 1977 Oct 15;82(2):187–190. doi: 10.1016/0014-5793(77)80581-4. [DOI] [PubMed] [Google Scholar]
- Osborn M., Person S., Phillips S., Funk F. A determination of mutagen specificity in bacteria using nonsense mutants of bacteriophage T4. J Mol Biol. 1967 Jun 28;26(3):437–447. doi: 10.1016/0022-2836(67)90314-2. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S., Revello P. T. Sensory adaptation mutants of E. coli. Cell. 1978 Dec;15(4):1221–1230. doi: 10.1016/0092-8674(78)90048-x. [DOI] [PubMed] [Google Scholar]
- Silverman M., Matsumura P., Simon M. The identification of the mot gene product with Escherichia coli-lambda hybrids. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3126–3130. doi: 10.1073/pnas.73.9.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S., Cragoe E. J., Jr, Imae Y. Amiloride, a specific inhibitor for the Na+-driven flagellar motors of alkalophilic Bacillus. J Biol Chem. 1988 Jun 15;263(17):8215–8219. [PubMed] [Google Scholar]
- Sugiyama S., Matsukura H., Imae Y. Relationship between Na+-dependent cytoplasmic pH homeostasis and Na+-dependent flagellar rotation and amino acid transport in alkalophilic Bacillus. FEBS Lett. 1985 Mar 25;182(2):265–268. doi: 10.1016/0014-5793(85)80312-4. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Asano M., Shimamura Y., Unemoto T., Sugiyama S., Imae Y. Roles of the respiratory Na+ pump in bioenergetics of Vibrio alginolyticus. J Biochem. 1988 Apr;103(4):650–655. doi: 10.1093/oxfordjournals.jbchem.a122323. [DOI] [PubMed] [Google Scholar]
- Uozumi T., Hoshino T., Miwa K., Horinouchi S., Beppu T., Arima K. Restriction and modification in Bacillus species: genetic transformation of bacteria with DNA from different species, part I. Mol Gen Genet. 1977 Mar 28;152(1):65–69. doi: 10.1007/BF00264941. [DOI] [PubMed] [Google Scholar]
- Wilson M. L., Macnab R. M. Overproduction of the MotA protein of Escherichia coli and estimation of its wild-type level. J Bacteriol. 1988 Feb;170(2):588–597. doi: 10.1128/jb.170.2.588-597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


