Abstract
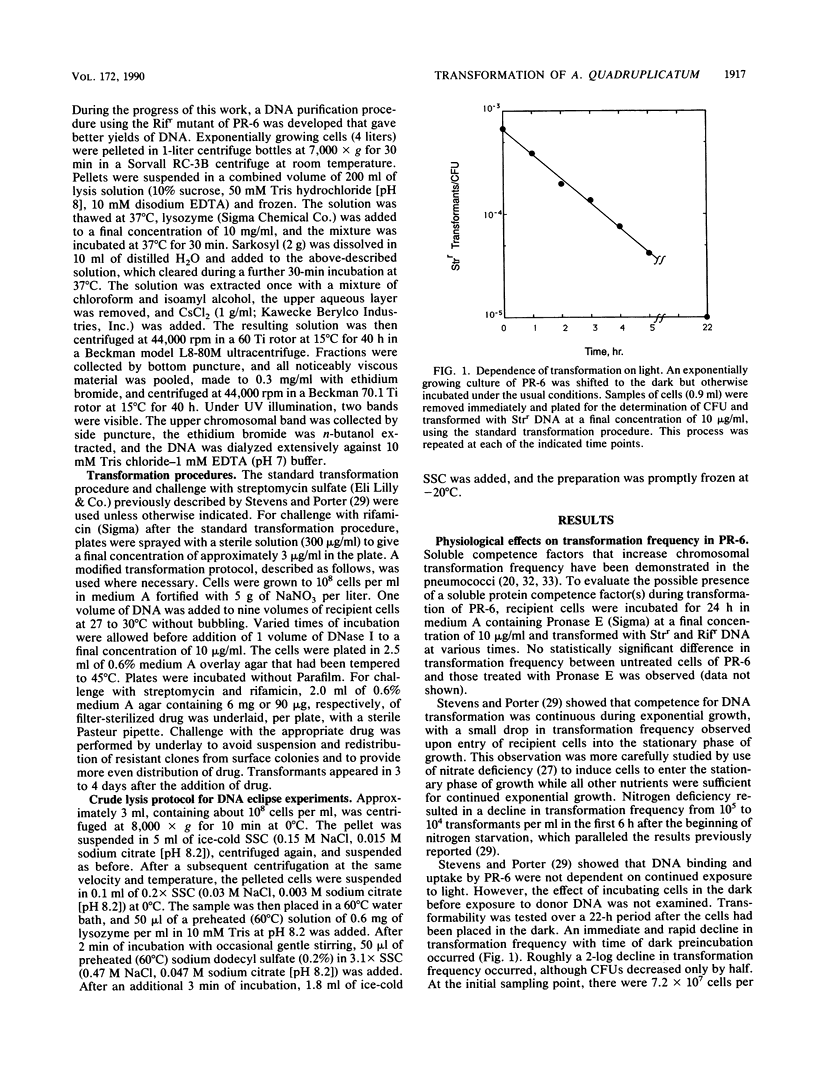
Chromosomal transformation of Agmenellum quadruplicatum PR-6 (= Synechococcus sp. strain 7002) was characterized for phenotypic expression, for exposure time to DNA, and for dependence on DNA concentration with regard to Rifr donor DNA. Exponentially growing cells of PR-6 were competent for chromosomal transformation. Competence decreased in cells in the stationary phase of growth or in cells deprived of a nitrogen source. Dark incubation of cells before exposure to donor DNA also decreased competence. Homologous Rifr and Strr DNA and heterologous Escherichia coli W3110 DNA were used in DNA-DNA competition studies, which clearly showed that DNA binding by PR-6 was nonspecific. DNA binding and uptake by PR-6 exhibited single-hit kinetics. Single-stranded DNA failed to transform competent cells of PR-6, and DNA eclipse was not observed, suggesting that double-stranded DNA was the substrate for the binding and uptake reactions during the transformation of PR-6. A significant improvement in transformation frequency was achieved by increasing the nitrate content of the culture medium and by lowering the temperature at which cells were exposed to donor DNA from 39 degrees C (the optimal temperature for growth) to 30 degrees C.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barany F., Kahn M. E., Smith H. O. Directional transport and integration of donor DNA in Haemophilus influenzae transformation. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7274–7278. doi: 10.1073/pnas.80.23.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G. D., Sox T., Blackman E., Sparling P. F. Factors affecting genetic transformation of Neisseria gonorrhoeae. J Bacteriol. 1977 Feb;129(2):983–992. doi: 10.1128/jb.129.2.983-992.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G. D., Sparling P. F. Entry of double-stranded deoxyribonucleic acid during transformation of Neisseria gonorrhoeae. J Bacteriol. 1981 Jan;145(1):638–640. doi: 10.1128/jb.145.1.638-640.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzby J. S., Porter R. D., Stevens S. E., Jr Expression of the Escherichia coli lacZ gene on a plasmid vector in a cyanobacterium. Science. 1985 Nov 15;230(4727):805–807. doi: 10.1126/science.2997920. [DOI] [PubMed] [Google Scholar]
- Buzby J. S., Porter R. D., Stevens S. E., Jr Plasmid transformation in Agmenellum quadruplicatum PR-6: construction of biphasic plasmids and characterization of their transformation properties. J Bacteriol. 1983 Jun;154(3):1446–1450. doi: 10.1128/jb.154.3.1446-1450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvat F., Astier C., Vedel F., Joset-Espardellier F. Transformation in the cyanobacterium Synechococcus R2: improvement of efficiency; role of the pUH24 plasmid. Mol Gen Genet. 1983;191(1):39–45. doi: 10.1007/BF00330887. [DOI] [PubMed] [Google Scholar]
- Danner D. B., Deich R. A., Sisco K. L., Smith H. O. An eleven-base-pair sequence determines the specificity of DNA uptake in Haemophilus transformation. Gene. 1980 Nov;11(3-4):311–318. doi: 10.1016/0378-1119(80)90071-2. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J., Asmus A., Tomasz A. Specificity of DNA uptake in genetic transformation of gonococci. Biochem Biophys Res Commun. 1979 Jan 15;86(1):97–104. doi: 10.1016/0006-291x(79)90386-3. [DOI] [PubMed] [Google Scholar]
- FOX M. S., HOTCHKISS R. D. Fate of transforming deoxyribonucleate following fixation by transformable bacteria. Nature. 1960 Sep 17;187:1002–1006. doi: 10.1038/1871002a0. [DOI] [PubMed] [Google Scholar]
- Ghei O. K., Lacks S. A. Recovery of donor deoxyribonucleic acid marker activity from eclipse in pneumococcal transformation. J Bacteriol. 1967 Mar;93(3):816–829. doi: 10.1128/jb.93.3.816-829.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Sherman L. A. Optimal conditions for genetic transformation of the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1984 Apr;158(1):36–42. doi: 10.1128/jb.158.1.36-42.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. E., Maul G., Goodgal S. H. Possible mechanism for donor DNA binding and transport in Haemophilus. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6370–6374. doi: 10.1073/pnas.79.20.6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S., TOLMACH L. J. Genetic transformation. I. Cellular incorporation of DNA accompanying transformation in Pneumococcus. Biochim Biophys Acta. 1957 Oct;26(1):68–82. doi: 10.1016/0006-3002(57)90055-0. [DOI] [PubMed] [Google Scholar]
- Low K. B., Porter D. D. Modes of gene transfer and recombination in bacteria. Annu Rev Genet. 1978;12:249–287. doi: 10.1146/annurev.ge.12.120178.001341. [DOI] [PubMed] [Google Scholar]
- Pakula R., Hauschild A. H. The effect of "competase" on DNA uptake in provoked transformation of a streptococcus. Can J Microbiol. 1965 Oct;11(5):823–827. doi: 10.1139/m65-111. [DOI] [PubMed] [Google Scholar]
- Piechowska M., Fox M. S. Fate of transforming deoxyribonucleate in Bacillus subtilis. J Bacteriol. 1971 Nov;108(2):680–689. doi: 10.1128/jb.108.2.680-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., Buzby J. S., Pilon A., Fields P. I., Dubbs J. M., Stevens S. E., Jr Genes from the cyanobacterium Agmenellum quadruplicatum isolated by complementation: characterization and production of merodiploids. Gene. 1986;41(2-3):249–260. doi: 10.1016/0378-1119(86)90105-8. [DOI] [PubMed] [Google Scholar]
- Porter R. D. Transformation in cyanobacteria. Crit Rev Microbiol. 1986;13(2):111–132. doi: 10.3109/10408418609108736. [DOI] [PubMed] [Google Scholar]
- Scocca J. J., Poland R. L., Zoon K. C. Specificity in deoxyribonucleic acid uptake by transformable Haemophilus influenzae. J Bacteriol. 1974 May;118(2):369–373. doi: 10.1128/jb.118.2.369-373.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestakov S. V., Khyen N. T. Evidence for genetic transformation in blue-green alga Anacystis nidulans. Mol Gen Genet. 1970;107(4):372–375. doi: 10.1007/BF00441199. [DOI] [PubMed] [Google Scholar]
- Soltyk A., Shugar D., Piechowska M. Heterologous deoxyribonucleic acid uptake and complexing with cellular constituents in competent Bacillus subtilis. J Bacteriol. 1975 Dec;124(3):1429–1438. doi: 10.1128/jb.124.3.1429-1438.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens S. E., Jr, Porter R. D. Heterospecific transformation among cyanobacteria. J Bacteriol. 1986 Sep;167(3):1074–1076. doi: 10.1128/jb.167.3.1074-1076.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
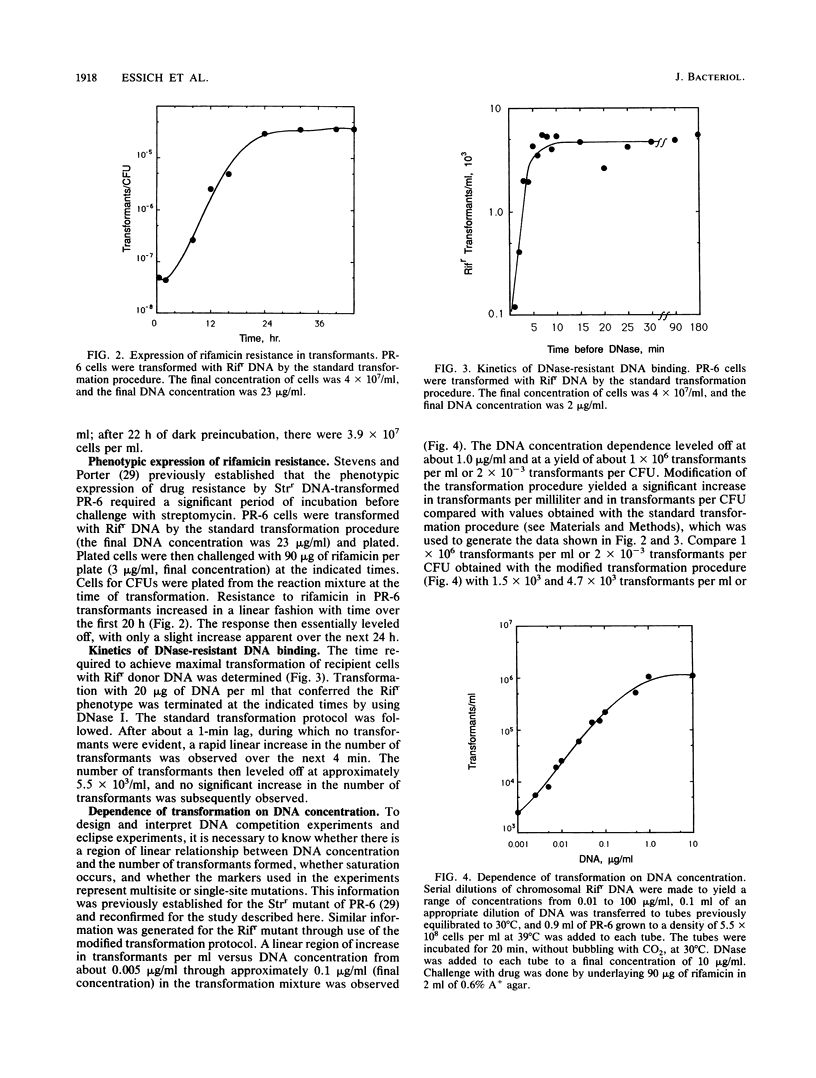
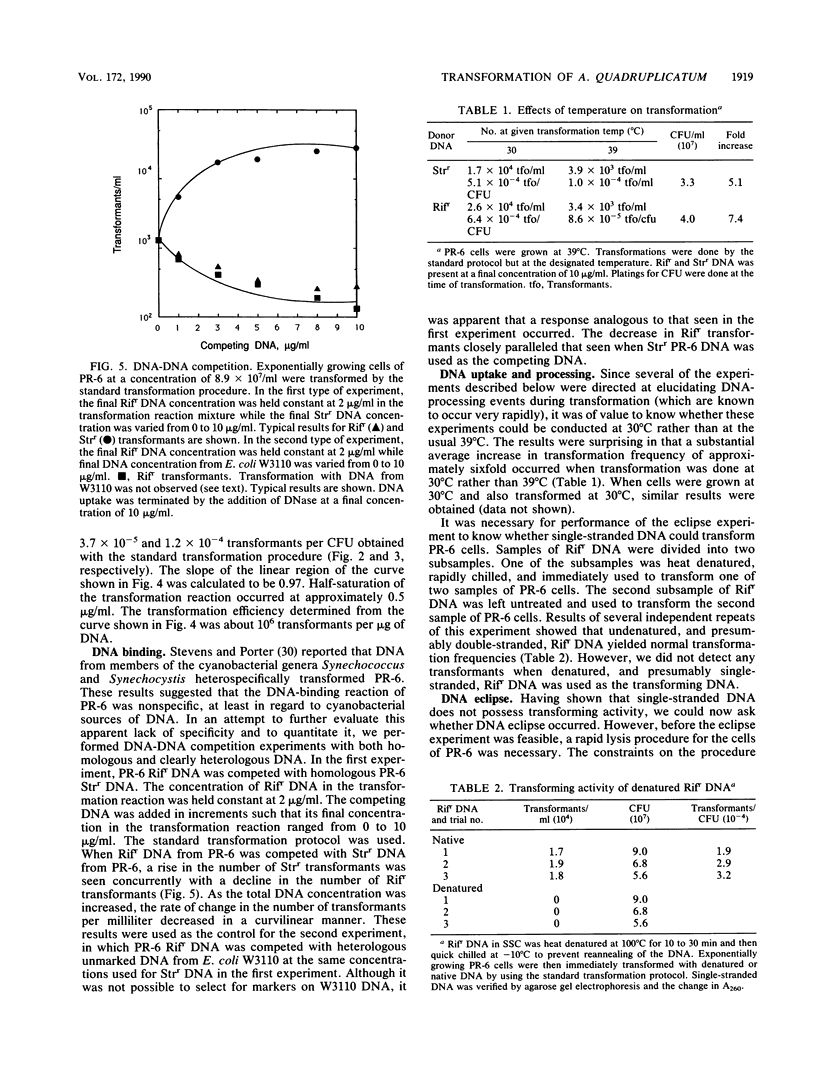
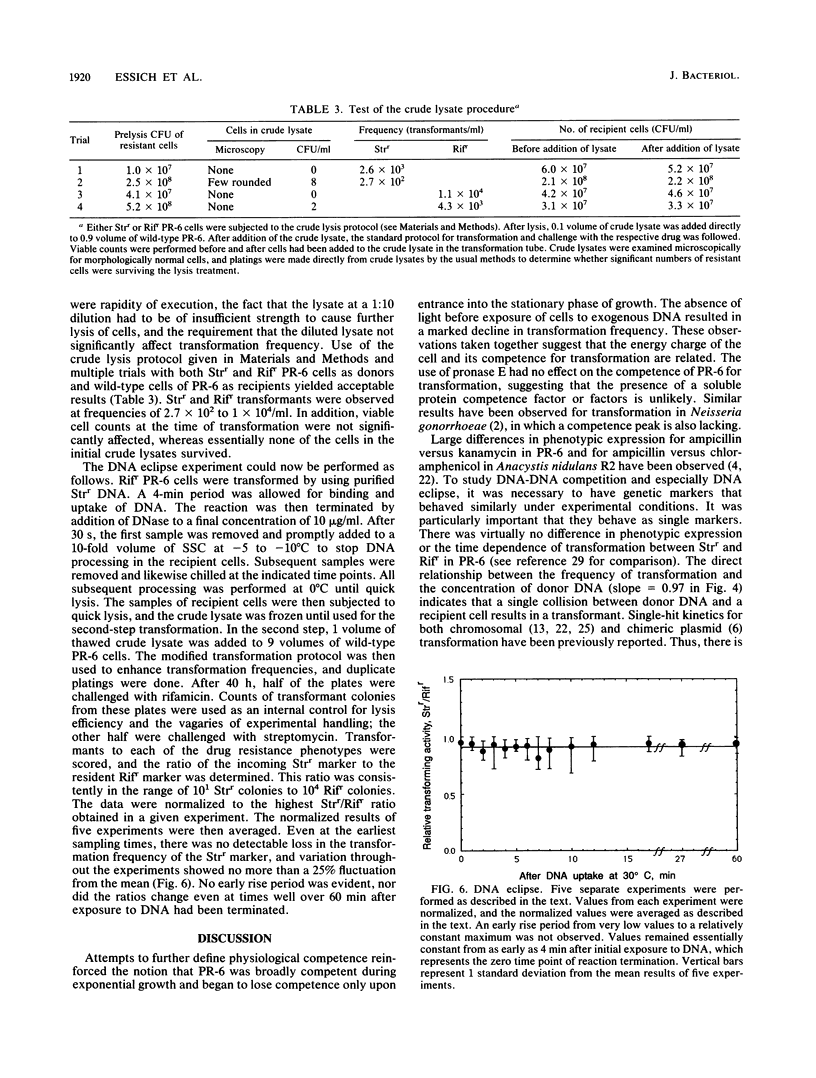
- Stevens S. E., Porter R. D. Transformation in Agmenellum quadruplicatum. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6052–6056. doi: 10.1073/pnas.77.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Fate of transforming DNA in the Haemophilus influenzae transformation system. J Mol Biol. 1965 Sep;13(2):554–570. doi: 10.1016/s0022-2836(65)80117-6. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Model for the mechanism controlling the expression of competent state in Pneumococcus cultures. J Bacteriol. 1966 Mar;91(3):1050–1061. doi: 10.1128/jb.91.3.1050-1061.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Mosser J. L. On the nature of the pneumococcal activator substance. Proc Natl Acad Sci U S A. 1966 Jan;55(1):58–66. doi: 10.1073/pnas.55.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENEMA G., PRITCHARD R. H., VENEMA-SCHROEDER T. FATE OF TRANSFORMING DEOXYRIBONUCLEIC ACID IN BACILLUS SUBTILIS. J Bacteriol. 1965 May;89:1250–1255. doi: 10.1128/jb.89.5.1250-1255.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLL M. J., GOODGAL S. H. Recombination during transformation in Hemophilus influenzae. Proc Natl Acad Sci U S A. 1961 Apr 15;47:505–512. doi: 10.1073/pnas.47.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorimier R., Guglielmi G., Bryant D. A., Stevens S. E., Jr Functional expression of plastid allophycocyanin genes in a cyanobacterium. J Bacteriol. 1987 May;169(5):1830–1835. doi: 10.1128/jb.169.5.1830-1835.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


