Abstract
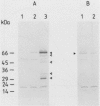
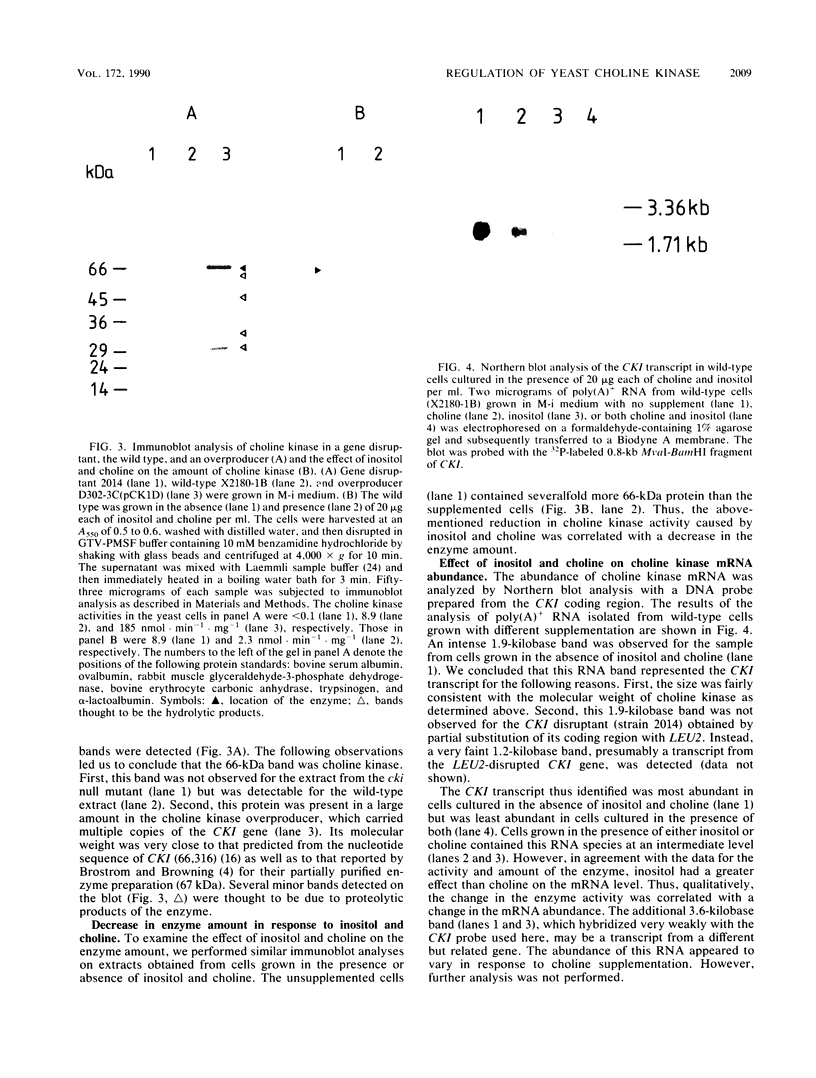
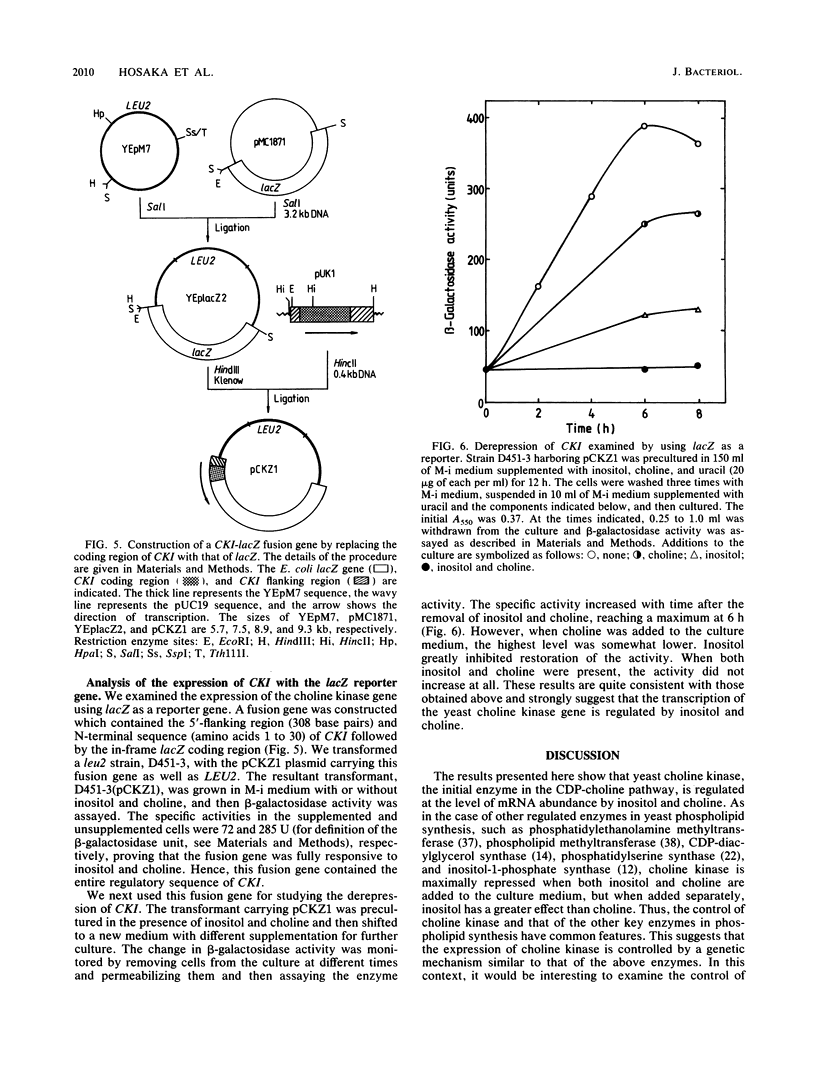
The regulation of choline kinase (EC 2.7.1.32), the initial enzyme in the CDP-choline pathway, was examined in Saccharomyces cerevisiae. The addition of myo-inositol to a culture of wild-type cells resulted in a significant decrease in choline kinase activity. Additional supplementation of choline caused a further reduction in the activity. The coding frame of the choline kinase gene, CK1, was joined to the carboxyl terminus of lacZ and expressed in Escherichia coli as a fusion protein, which was then used to prepare an anti-choline kinase antibody. Upon Western (immuno-) and Northern (RNA) blot analyses using the antibody and a CK1 probe, respectively, the decrease in the enzyme activity was found to be correlated with decreases in the enzyme amount and mRNA abundance. The molecular mass of the enzyme was estimated to be 66 kilodaltons, in agreement with the value predicted previously from the nucleotide sequence of the gene. The coding region of CK1 was replaced with that of lacZ, and CK1 expression was measured by assaying beta-galactosidase. The expression of beta-galactosidase from this fusion was repressed by myo-inositol and choline and derepressed in a time-dependent manner upon their removal. The present findings indicate that yeast choline kinase is regulated by myo-inositol and choline at the level of mRNA abundance.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailis A. M., Poole M. A., Carman G. M., Henry S. A. The membrane-associated enzyme phosphatidylserine synthase is regulated at the level of mRNA abundance. Mol Cell Biol. 1987 Jan;7(1):167–176. doi: 10.1128/mcb.7.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Brostrom M. A., Browning E. T. Choline kinase from brewers' yeast. Partial purification, properties, and kinetic mechanism. J Biol Chem. 1973 Apr 10;248(7):2364–2371. [PubMed] [Google Scholar]
- Carson M. A., Atkinson K. D., Waechter C. J. Properties of particulate and solubilized phosphatidylserine synthase activity from Saccharomyces cerevisiae. Inhibitory effect of choline in the growth medium. J Biol Chem. 1982 Jul 25;257(14):8115–8121. [PubMed] [Google Scholar]
- Carson M. A., Emala M., Hogsten P., Waechter C. J. Coordinate regulation of phosphatidylserine decarboxylase activity and phospholipid N-methylation in yeast. J Biol Chem. 1984 May 25;259(10):6267–6273. [PubMed] [Google Scholar]
- Culbertson M. R., Donahue T. F., Henry S. A. Control of inositol biosynthesis in Saccharomyces cerevisiae: properties of a repressible enzyme system in extracts of wild-type (Ino+) cells. J Bacteriol. 1976 Apr;126(1):232–242. doi: 10.1128/jb.126.1.232-242.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue T. F., Henry S. A. myo-Inositol-1-phosphate synthase. Characteristics of the enzyme and identification of its structural gene in yeast. J Biol Chem. 1981 Jul 10;256(13):7077–7085. [PubMed] [Google Scholar]
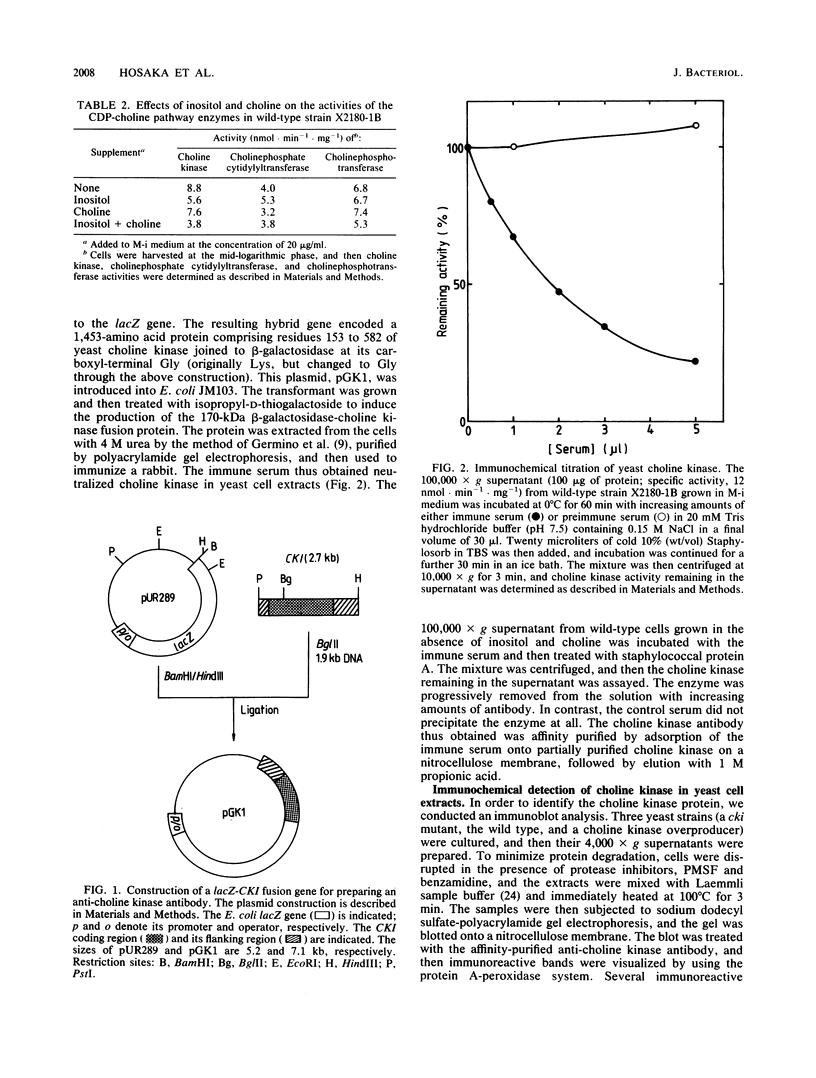
- Germino J., Gray J. G., Charbonneau H., Vanaman T., Bastia D. Use of gene fusions and protein-protein interaction in the isolation of a biologically active regulatory protein: the replication initiator protein of plasmid R6K. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6848–6852. doi: 10.1073/pnas.80.22.6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. L., Hubbell S., Lam C. Inositol regulates phosphatidylglycerolphosphate synthase expression in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Nov;8(11):4773–4779. doi: 10.1128/mcb.8.11.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. L., Donelson J. E. Nucleotide sequence of the yeast plasmid. Nature. 1980 Aug 28;286(5776):860–865. doi: 10.1038/286860a0. [DOI] [PubMed] [Google Scholar]
- Hirsch J. P., Henry S. A. Expression of the Saccharomyces cerevisiae inositol-1-phosphate synthase (INO1) gene is regulated by factors that affect phospholipid synthesis. Mol Cell Biol. 1986 Oct;6(10):3320–3328. doi: 10.1128/mcb.6.10.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann M. J., Bailis A. M., Henry S. A., Carman G. M. Coordinate regulation of phospholipid biosynthesis by serine in Saccharomyces cerevisiae. J Bacteriol. 1987 Jul;169(7):3276–3280. doi: 10.1128/jb.169.7.3276-3280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann M. J., Henry S. A., Carman G. M. Regulation of CDP-diacylglycerol synthase activity in Saccharomyces cerevisiae. J Bacteriol. 1985 Sep;163(3):1265–1266. doi: 10.1128/jb.163.3.1265-1266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann M. J., Poole M. A., Gaynor P. M., Ho C. T., Carman G. M. Effect of growth phase on phospholipid biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1987 Feb;169(2):533–539. doi: 10.1128/jb.169.2.533-539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K., Kodaki T., Yamashita S. Cloning and characterization of the yeast CKI gene encoding choline kinase and its expression in Escherichia coli. J Biol Chem. 1989 Feb 5;264(4):2053–2059. [PubMed] [Google Scholar]
- Hosaka K., Yamashita S. Choline transport in Saccharomyces cerevisiae. J Bacteriol. 1980 Jul;143(1):176–181. doi: 10.1128/jb.143.1.176-181.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverius P. H. Coupling of glycosaminoglycans to agarose beads (sepharose 4B). Biochem J. 1971 Oct;124(4):677–683. doi: 10.1042/bj1240677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T., Broek D., Wigler M. DNA sequence and characterization of the S. cerevisiae gene encoding adenylate cyclase. Cell. 1985 Dec;43(2 Pt 1):493–505. doi: 10.1016/0092-8674(85)90179-5. [DOI] [PubMed] [Google Scholar]
- Kelley M. J., Bailis A. M., Henry S. A., Carman G. M. Regulation of phospholipid biosynthesis in Saccharomyces cerevisiae by inositol. Inositol is an inhibitor of phosphatidylserine synthase activity. J Biol Chem. 1988 Dec 5;263(34):18078–18085. [PubMed] [Google Scholar]
- Kinney A. J., Carman G. M. Phosphorylation of yeast phosphatidylserine synthase in vivo and in vitro by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7962–7966. doi: 10.1073/pnas.85.21.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klig L. S., Homann M. J., Carman G. M., Henry S. A. Coordinate regulation of phospholipid biosynthesis in Saccharomyces cerevisiae: pleiotropically constitutive opi1 mutant. J Bacteriol. 1985 Jun;162(3):1135–1141. doi: 10.1128/jb.162.3.1135-1141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodaki T., Yamashita S. Yeast phosphatidylethanolamine methylation pathway. Cloning and characterization of two distinct methyltransferase genes. J Biol Chem. 1987 Nov 15;262(32):15428–15435. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loewy B. S., Henry S. A. The INO2 and INO4 loci of Saccharomyces cerevisiae are pleiotropic regulatory genes. Mol Cell Biol. 1984 Nov;4(11):2479–2485. doi: 10.1128/mcb.4.11.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlock K. R., Lin Y. P., Carman G. M. Regulation of phosphatidate phosphatase activity by inositol in Saccharomyces cerevisiae. J Bacteriol. 1988 Aug;170(8):3561–3566. doi: 10.1128/jb.170.8.3561-3566.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa J., Sass P., Wigler M. Cloning and characterization of the low-affinity cyclic AMP phosphodiesterase gene of Saccharomyces cerevisiae. Mol Cell Biol. 1987 Oct;7(10):3629–3636. doi: 10.1128/mcb.7.10.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa J., Yonemura K., Yamashita S. Yeast mutant with thermolabile CDP-choline synthesis. Isolation and characterization of a cholinephosphate cytidyltransferase mutant. Eur J Biochem. 1983 Mar 1;131(1):223–229. doi: 10.1111/j.1432-1033.1983.tb07253.x. [DOI] [PubMed] [Google Scholar]
- Poole M. A., Homann M. J., Bae-Lee M. S., Carman G. M. Regulation of phosphatidylserine synthase from Saccharomyces cerevisiae by phospholipid precursors. J Bacteriol. 1986 Nov;168(2):668–672. doi: 10.1128/jb.168.2.668-672.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzkin B., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):487–491. doi: 10.1073/pnas.74.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter C. J., Lester R. L. Regulation of phosphatidylcholine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1971 Mar;105(3):837–843. doi: 10.1128/jb.105.3.837-843.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter C. J., Steiner M. R., Lester R. L. Regulation of phosphatidylcholine biosynthesis by the methylation pathway in Saccharomyces cerevisiae. J Biol Chem. 1969 Jun 25;244(12):3419–3422. [PubMed] [Google Scholar]
- Yamashita S., Oshima A., Nikawa J., Hosaka K. Regulation of the phosphatidylethanolamine methylation pathway in Saccharomyces cerevisiae. Eur J Biochem. 1982 Nov 15;128(2-3):589–595. doi: 10.1111/j.1432-1033.1982.tb07005.x. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Oshima A. Regulation of phosphatidylethanolamine methyltransferase level by myo-inositol in Saccaromyces cerevisiae. Eur J Biochem. 1980 Mar;104(2):611–616. doi: 10.1111/j.1432-1033.1980.tb04465.x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]