Abstract
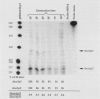
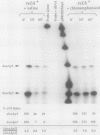
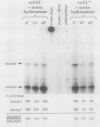
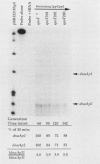
Two promoters for the Escherichia coli operon that contains the four genes dnaA, dnaN, recF, and gyrB were found to be growth rate regulated and under stringent control. Transcript abundance relative to total RNA increased with the growth rate. Changes in transcription from the dnaAp1 and dnaAp2 promoters that were induced by amino acid starvation and chloramphenicol and were relA dependent were correlated with the stringent response. The abundance of these transcripts per total RNA also decreased in spoT mutants as the severity of the mutation increased (guanosine 5'-diphosphate 3'-diphosphate [ppGpp] basal levels increased). Because expression of these promoters appears to be inhibited by ppGpp, it is proposed that one mechanism for coupling DNA replication to the growth rate of bacteria is through ppGpp synthesis at the ribosome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Mizuuchi K., Menzel R., Gellert M. DNA sequence and transcription of the region upstream of the E. coli gyrB gene. Nucleic Acids Res. 1984 Aug 24;12(16):6389–6395. doi: 10.1093/nar/12.16.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi T., Mizuuchi M., Robinson E. A., Appella E., O'Dea M. H., Gellert M., Mizuuchi K. DNA sequence of the E. coli gyrB gene: application of a new sequencing strategy. Nucleic Acids Res. 1987 Jan 26;15(2):771–784. doi: 10.1093/nar/15.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armengod M. E., García-Sogo M., Lambíes E. Transcriptional organization of the dnaN and recF genes of Escherichia coli K-12. J Biol Chem. 1988 Aug 25;263(24):12109–12114. [PubMed] [Google Scholar]
- Armengod M. E., Lambíes E. Overlapping arrangement of the recF and dnaN operons of Escherichia coli; positive and negative control sequences. Gene. 1986;43(3):183–196. doi: 10.1016/0378-1119(86)90206-4. [DOI] [PubMed] [Google Scholar]
- Atlung T., Clausen E. S., Hansen F. G. Autoregulation of the dnaA gene of Escherichia coli K12. Mol Gen Genet. 1985;200(3):442–450. doi: 10.1007/BF00425729. [DOI] [PubMed] [Google Scholar]
- Baracchini E., Bremer H. Stringent and growth control of rRNA synthesis in Escherichia coli are both mediated by ppGpp. J Biol Chem. 1988 Feb 25;263(6):2597–2602. [PubMed] [Google Scholar]
- Blanar M. A., Sandler S. J., Armengod M. E., Ream L. W., Clark A. J. Molecular analysis of the recF gene of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4622–4626. doi: 10.1073/pnas.81.15.4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. A model for initiation at origins of DNA replication. Cell. 1988 Sep 23;54(7):915–918. doi: 10.1016/0092-8674(88)90102-x. [DOI] [PubMed] [Google Scholar]
- Braun R. E., O'Day K., Wright A. Autoregulation of the DNA replication gene dnaA in E. coli K-12. Cell. 1985 Jan;40(1):159–169. doi: 10.1016/0092-8674(85)90319-8. [DOI] [PubMed] [Google Scholar]
- Chiaramello A. E., Zyskind J. W. Expression of Escherichia coli dnaA and mioC genes as a function of growth rate. J Bacteriol. 1989 Aug;171(8):4272–4280. doi: 10.1128/jb.171.8.4272-4280.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Dickson R. R., Gaal T., deBoer H. A., deHaseth P. L., Gourse R. L. Identification of promoter mutants defective in growth-rate-dependent regulation of rRNA transcription in Escherichia coli. J Bacteriol. 1989 Sep;171(9):4862–4870. doi: 10.1128/jb.171.9.4862-4870.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourse R. L., Stark M. J., Dahlberg A. E. Regions of DNA involved in the stringent control of plasmid-encoded rRNA in vivo. Cell. 1983 Apr;32(4):1347–1354. doi: 10.1016/0092-8674(83)90315-x. [DOI] [PubMed] [Google Scholar]
- Gourse R. L., de Boer H. A., Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell. 1986 Jan 17;44(1):197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- Hansen E. B., Hansen F. G., von Meyenburg K. The nucleotide sequence of the dnaA gene and the first part of the dnaN gene of Escherichia coli K-12. Nucleic Acids Res. 1982 Nov 25;10(22):7373–7385. doi: 10.1093/nar/10.22.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F. G., Hansen E. B., Atlung T. The nucleotide sequence of the dnaA gene promoter and of the adjacent rpmH gene, coding for the ribosomal protein L34, of Escherichia coli. EMBO J. 1982;1(9):1043–1048. doi: 10.1002/j.1460-2075.1982.tb01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemeyer E. A., Richter D. Characterization of the guanosine 5'-triphosphate 3'-diphosphate and guanosine 5'-diphosphate 3'-diphosphate degradation reaction catalyzed by a specific pyrophosphorylase from Escherichia coli. Biochemistry. 1978 Dec 12;17(25):5368–5372. doi: 10.1021/bi00618a007. [DOI] [PubMed] [Google Scholar]
- Kücherer C., Lother H., Kölling R., Schauzu M. A., Messer W. Regulation of transcription of the chromosomal dnaA gene of Escherichia coli. Mol Gen Genet. 1986 Oct;205(1):115–121. doi: 10.1007/BF02428040. [DOI] [PubMed] [Google Scholar]
- Lagosky P. A., Chang F. N. Correlation between RNA synthesis and basal level guanosine 5'-diphosphate 3'-diphosphate in relaxed mutants of Escherichia coli. J Biol Chem. 1981 Nov 25;256(22):11651–11656. [PubMed] [Google Scholar]
- Lagosky P. A., Chang F. N. Influence of amino acid starvation on guanosine 5'-diphosphate 3'-diphosphate basal-level synthesis in Escherichia coli. J Bacteriol. 1980 Nov;144(2):499–508. doi: 10.1128/jb.144.2.499-508.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond A. I., Travers A. A. Genetically separable functional elements mediate the optimal expression and stringent regulation of a bacterial tRNA gene. Cell. 1985 Feb;40(2):319–326. doi: 10.1016/0092-8674(85)90146-1. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Løbner-Olesen A., Atlung T., Rasmussen K. V. Stability and replication control of Escherichia coli minichromosomes. J Bacteriol. 1987 Jun;169(6):2835–2842. doi: 10.1128/jb.169.6.2835-2842.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løbner-Olesen A., Skarstad K., Hansen F. G., von Meyenburg K., Boye E. The DnaA protein determines the initiation mass of Escherichia coli K-12. Cell. 1989 Jun 2;57(5):881–889. doi: 10.1016/0092-8674(89)90802-7. [DOI] [PubMed] [Google Scholar]
- Madiraju M. V., Templin A., Clark A. J. Properties of a mutant recA-encoded protein reveal a possible role for Escherichia coli recF-encoded protein in genetic recombination. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6592–6596. doi: 10.1073/pnas.85.18.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffy J. M., Zyskind J. W. A model for the initiation of replication in Escherichia coli. J Theor Biol. 1989 Oct 23;140(4):453–477. doi: 10.1016/s0022-5193(89)80109-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McHenry C. S. DNA polymerase III holoenzyme of Escherichia coli. Annu Rev Biochem. 1988;57:519–550. doi: 10.1146/annurev.bi.57.070188.002511. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Kimura M., Nagata T., Sakakibara Y. Structural analysis of the dnaA and dnaN genes of Escherichia coli. Gene. 1984 May;28(2):159–170. doi: 10.1016/0378-1119(84)90253-1. [DOI] [PubMed] [Google Scholar]
- Pedersen F. S., Lund E., Kjeldgaard N. O. Codon specific, tRNA dependent in vitro synthesis of ppGpp and pppGpp. Nat New Biol. 1973 May 2;243(122):13–15. [PubMed] [Google Scholar]
- Pierucci O., Rickert M., Helmstetter C. E. DnaA protein overproduction abolishes cell cycle specificity of DNA replication from oriC in Escherichia coli. J Bacteriol. 1989 Jul;171(7):3760–3766. doi: 10.1128/jb.171.7.3760-3766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones A., Kaasch J., Kaasch M., Messer W. Induction of dnaN and dnaQ gene expression in Escherichia coli by alkylation damage to DNA. EMBO J. 1989 Feb;8(2):587–593. doi: 10.1002/j.1460-2075.1989.tb03413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones A., Messer W. Discoordinate gene expression in the dnaA-dnaN operon of Escherichia coli. Mol Gen Genet. 1988 Jul;213(1):118–124. doi: 10.1007/BF00333407. [DOI] [PubMed] [Google Scholar]
- Quiñones A., Piechocki R., Messer W. Expression of the Escherichia coli dnaQ (mutD) gene is inducible. Mol Gen Genet. 1988 Jan;211(1):106–112. doi: 10.1007/BF00338400. [DOI] [PubMed] [Google Scholar]
- Rokeach L. A., Kassavetis G. A., Zyskind J. W. RNA polymerase pauses in vitro within the Escherichia coli origin of replication at the same sites where termination occurs in vivo. J Biol Chem. 1987 May 25;262(15):7264–7272. [PubMed] [Google Scholar]
- Rokeach L. A., Zyskind J. W. RNA terminating within the E. coli origin of replication: stringent regulation and control by DnaA protein. Cell. 1986 Aug 29;46(5):763–771. doi: 10.1016/0092-8674(86)90352-1. [DOI] [PubMed] [Google Scholar]
- Ryals J., Little R., Bremer H. Control of rRNA and tRNA syntheses in Escherichia coli by guanosine tetraphosphate. J Bacteriol. 1982 Sep;151(3):1261–1268. doi: 10.1128/jb.151.3.1261-1268.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y., Tsukano H., Sako T. Organization and transcription of the dnaA and dnaN genes of Escherichia coli. Gene. 1981 Jan-Feb;13(1):47–55. doi: 10.1016/0378-1119(81)90042-1. [DOI] [PubMed] [Google Scholar]
- Sako T., Sakakibara Y. Coordinate expression of Escherichia coli dnaA and dnaN genes. Mol Gen Genet. 1980;179(3):521–526. doi: 10.1007/BF00271741. [DOI] [PubMed] [Google Scholar]
- Sarmientos P., Cashel M. Carbon starvation and growth rate-dependent regulation of the Escherichia coli ribosomal RNA promoters: differential control of dual promoters. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7010–7013. doi: 10.1073/pnas.80.22.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmientos P., Sylvester J. E., Contente S., Cashel M. Differential stringent control of the tandem E. coli ribosomal RNA promoters from the rrnA operon expressed in vivo in multicopy plasmids. Cell. 1983 Apr;32(4):1337–1346. doi: 10.1016/0092-8674(83)90314-8. [DOI] [PubMed] [Google Scholar]
- Sarubbi E., Rudd K. E., Cashel M. Basal ppGpp level adjustment shown by new spoT mutants affect steady state growth rates and rrnA ribosomal promoter regulation in Escherichia coli. Mol Gen Genet. 1988 Aug;213(2-3):214–222. doi: 10.1007/BF00339584. [DOI] [PubMed] [Google Scholar]
- Schaefer C., Messer W. Directionality of DnaA protein/DNA interaction. Active orientation of the DnaA protein/dnaA box complex in transcription termination. EMBO J. 1989 May;8(5):1609–1613. doi: 10.1002/j.1460-2075.1989.tb03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimizu K., Bramhill D., Kornberg A. ATP activates dnaA protein in initiating replication of plasmids bearing the origin of the E. coli chromosome. Cell. 1987 Jul 17;50(2):259–265. doi: 10.1016/0092-8674(87)90221-2. [DOI] [PubMed] [Google Scholar]
- Sekimizu K., Kornberg A. Cardiolipin activation of dnaA protein, the initiation protein of replication in Escherichia coli. J Biol Chem. 1988 May 25;263(15):7131–7135. [PubMed] [Google Scholar]
- Shand R. F., Blum P. H., Mueller R. D., Riggs D. L., Artz S. W. Correlation between histidine operon expression and guanosine 5'-diphosphate-3'-diphosphate levels during amino acid downshift in stringent and relaxed strains of Salmonella typhimurium. J Bacteriol. 1989 Feb;171(2):737–743. doi: 10.1128/jb.171.2.737-743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosa T., Pizer L. I. Biochemical bases for the antimetabolite action of L-serine hydroxamate. J Bacteriol. 1971 Jun;106(3):972–982. doi: 10.1128/jb.106.3.972-982.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A. Conserved features of coordinately regulated E. coli promoters. Nucleic Acids Res. 1984 Mar 26;12(6):2605–2618. doi: 10.1093/nar/12.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. Modulation of RNA polymerase specificity by ppGpp. Mol Gen Genet. 1976 Aug 19;147(2):225–232. doi: 10.1007/BF00267575. [DOI] [PubMed] [Google Scholar]
- Travers A. RNA polymerase specificity and the control of growth. Nature. 1976 Oct 21;263(5579):641–646. doi: 10.1038/263641a0. [DOI] [PubMed] [Google Scholar]
- Wang Q. P., Kaguni J. M. Transcriptional repression of the dnaA gene of Escherichia coli by dnaA protein. Mol Gen Genet. 1987 Oct;209(3):518–525. doi: 10.1007/BF00331158. [DOI] [PubMed] [Google Scholar]
- Yamagishi M., Cole J. R., Nomura M., Studier F. W., Dunn J. J. Stringent control in Escherichia coli applies also to transcription by T7 RNA polymerase. J Biol Chem. 1987 Mar 25;262(9):3940–3943. [PubMed] [Google Scholar]