Abstract
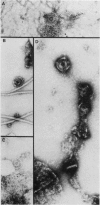
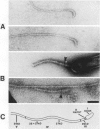
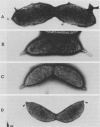
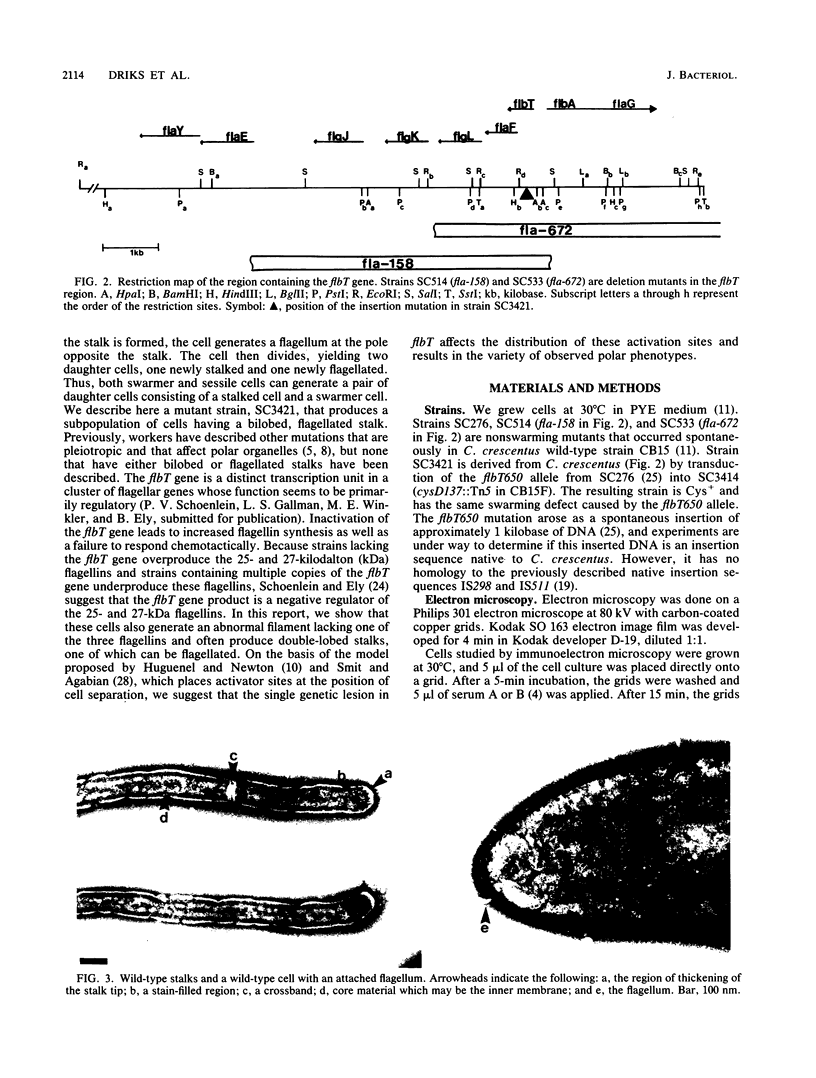
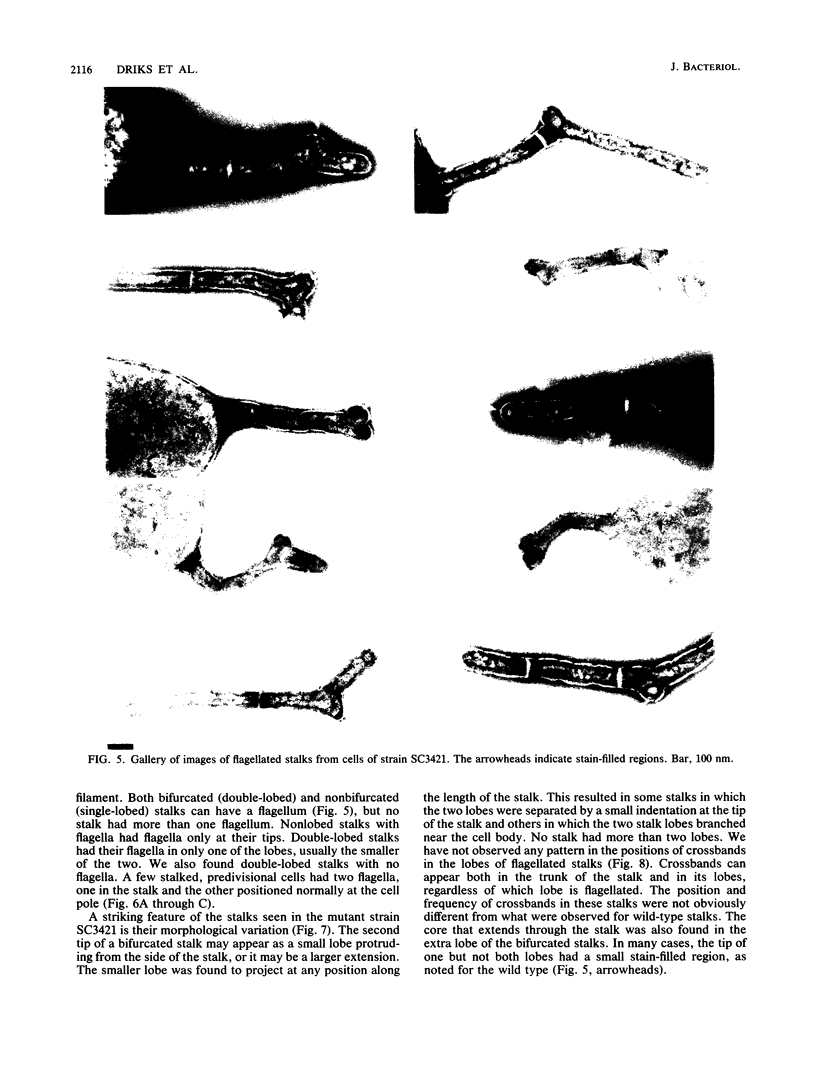
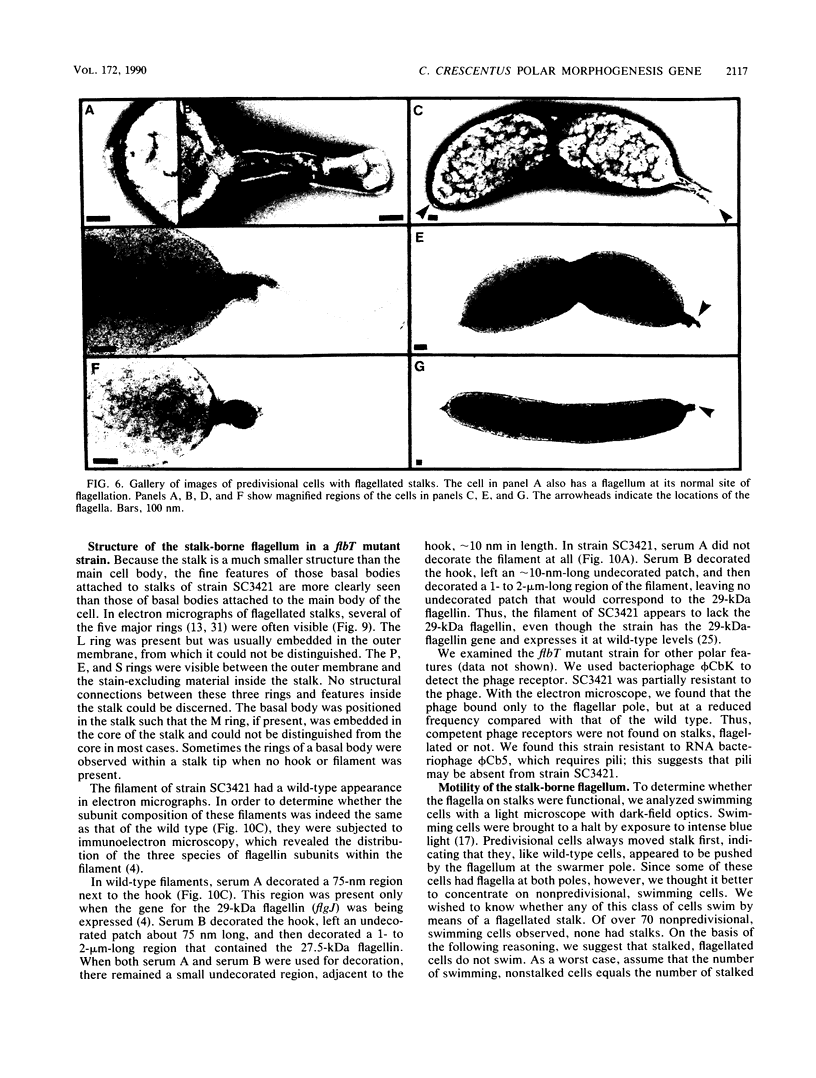
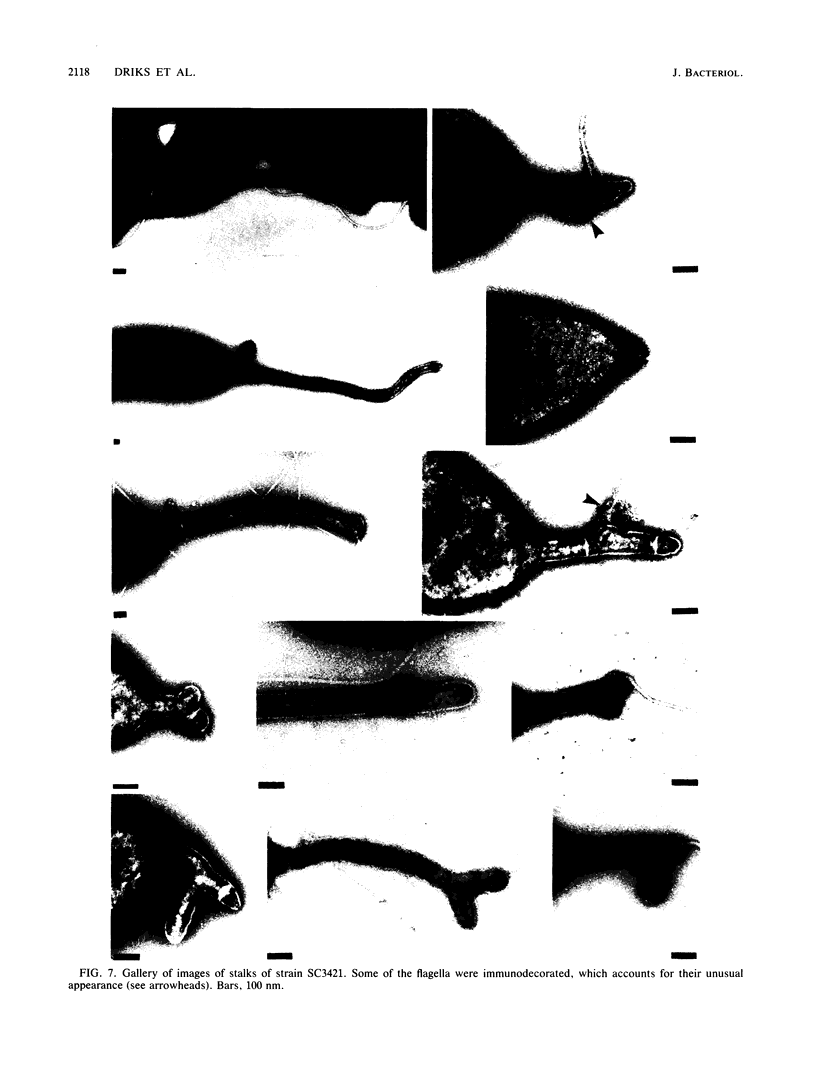
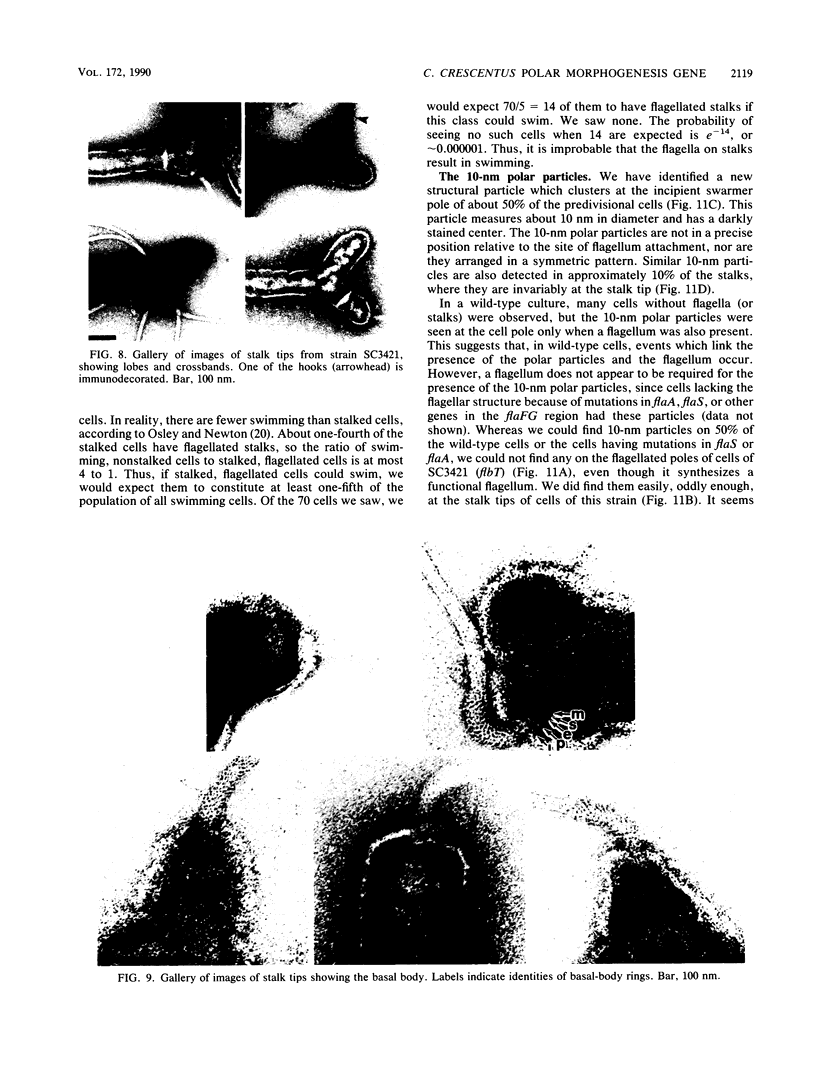
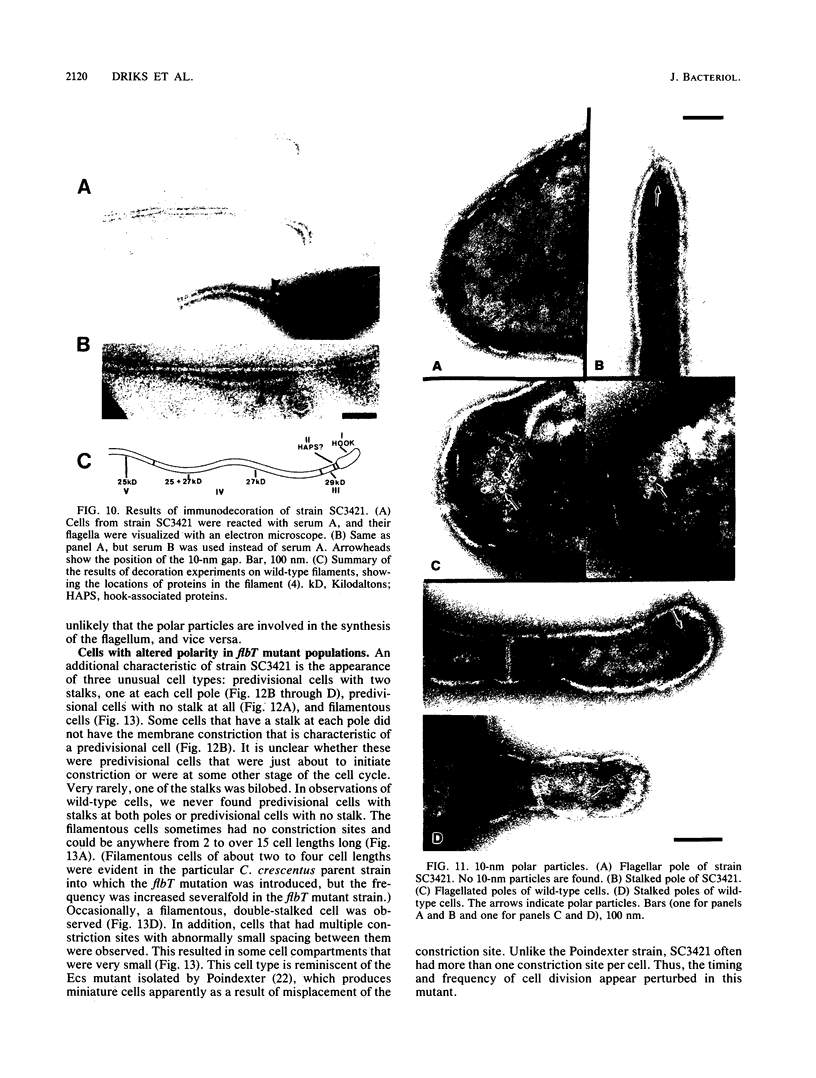
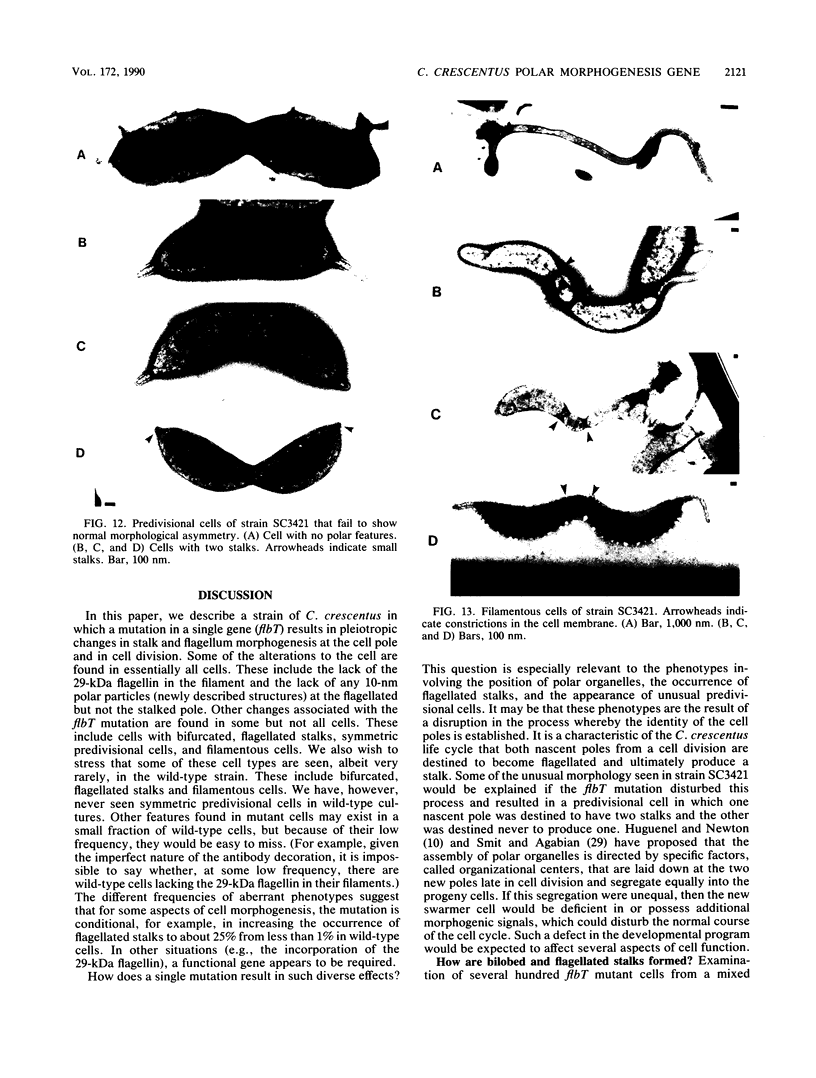
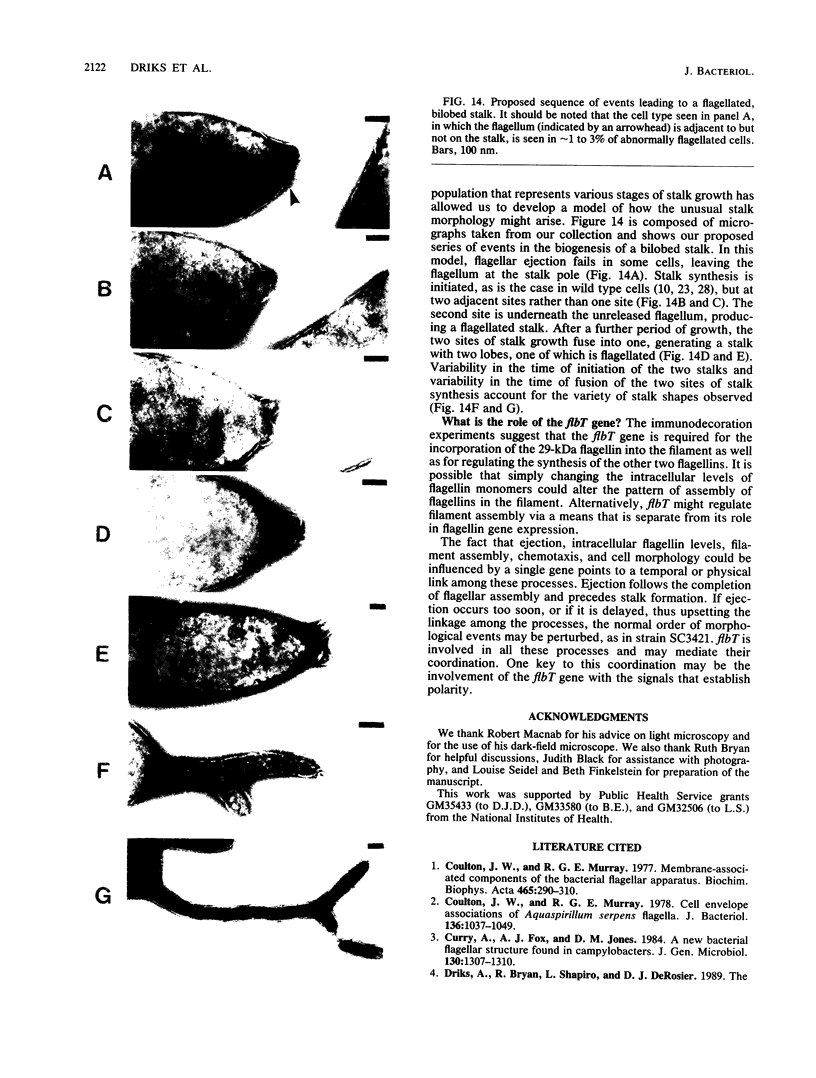
At specific times in the cell cycle, the bacterium Caulobacter crescentus assembles two major polar organelles, the flagellum and the stalk. Previous studies have shown that flbT mutants overproduce flagellins and are unable to form chemotaxis swarm rings. In this paper, we report alterations in both the stalk and the flagellar structure that result from a mutation in the flagellar gene flbT. Mutant strains produce some stalks that have a flagellum, produce some stalks that have an extra lobe protruding from their sides, have filaments lacking the 29-kilodalton flagellin, and produce several unusual cell types, including filamentous cells as well as predivisional cells with two stalks and predivisional cells with no stalk at all. We propose that flagellated stalks arise as a consequence of a failure to eject the flagellum at the correct time in the cell cycle and that the extra stalk lobe is due to a second site for the initiation of stalk biogenesis. Thus, a step in the pathway that establishes the characteristic asymmetry of the C. crescentus cell appears to be disrupted in flbT mutants. We have also identified a new structural feature at the flagellated pole and the tip of the stalk: the 10-nm polar particle. The polar particles appear as a cluster of approximately 1 to 10 stain-excluding rings, visible in electron micrographs of negatively stained wild-type cells. This structure is absent at the flagellar pole but not in the stalks of flbT mutant predivisional cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coulton J. W., Murray R. G. Cell envelope associations of Aquaspirillum serpens flagella. J Bacteriol. 1978 Dec;136(3):1037–1049. doi: 10.1128/jb.136.3.1037-1049.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Murray R. G. Membrane-associated components of the bacterial flagellar apparatus. Biochim Biophys Acta. 1977 Mar 1;465(2):290–310. doi: 10.1016/0005-2736(77)90080-3. [DOI] [PubMed] [Google Scholar]
- Curry A., Fox A. J., Jones D. M. A new bacterial flagellar structure found in campylobacters. J Gen Microbiol. 1984 May;130(5):1307–1310. doi: 10.1099/00221287-130-5-1307. [DOI] [PubMed] [Google Scholar]
- Ely B., Croft R. H., Gerardot C. J. Genetic mapping of genes required for motility in Caulobacter crescentus. Genetics. 1984 Nov;108(3):523–532. doi: 10.1093/genetics/108.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B., Gerardot C. J. Use of pulsed-field-gradient gel electrophoresis to construct a physical map of the Caulobacter crescentus genome. Gene. 1988 Sep 7;68(2):323–333. doi: 10.1016/0378-1119(88)90035-2. [DOI] [PubMed] [Google Scholar]
- Ferris F. G., Beveridge T. J., Marceau-Day M. L., Larson A. D. Structure and cell envelope associations of flagellar basal complexes of Vibrio cholerae and Campylobacter fetus. Can J Microbiol. 1984 Mar;30(3):322–333. doi: 10.1139/m84-048. [DOI] [PubMed] [Google Scholar]
- Fukuda A., Asada M., Koyasu S., Yoshida H., Yaginuma K., Okada Y. Regulation of polar morphogenesis in Caulobacter crescentus. J Bacteriol. 1981 Jan;145(1):559–572. doi: 10.1128/jb.145.1.559-572.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnenberger K. M., Shapiro L. Identification of a gene cluster involved in flagellar basal body biogenesis in Caulobacter crescentus. J Mol Biol. 1987 Mar 5;194(1):91–103. doi: 10.1016/0022-2836(87)90718-2. [DOI] [PubMed] [Google Scholar]
- Huguenel E. D., Newton A. Localization of surface structures during procaryotic differentiation: role of cell division in Caulobacter crescentus. Differentiation. 1982;21(2):71–78. doi: 10.1111/j.1432-0436.1982.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Analysis of nonmotile mutants of the dimorphic bacterium Caulobacter crescentus. J Bacteriol. 1979 Jan;137(1):627–634. doi: 10.1128/jb.137.1.627-634.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Isolation of spontaneously derived mutants of Caulobacter crescentus. Genetics. 1977 May;86(1):25–32. doi: 10.1093/genetics/86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Walsh M. P., Ely B., Shapiro L. Flagellar hook and basal complex of Caulobacter crescentus. J Bacteriol. 1979 Jun;138(3):984–989. doi: 10.1128/jb.138.3.984-989.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. C., Schmidt J. M. Ultrastructural study of crossbands occurring in the stalks of Caulobacter crescentus. J Bacteriol. 1973 Oct;116(1):466–470. doi: 10.1128/jb.116.1.466-470.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper J., Wildhaber I., Gao Z., Baeuerlein E. Basal-body-associated disks are additional structural elements of the flagellar apparatus isolated from Wolinella succinogenes. J Bacteriol. 1989 May;171(5):2803–2810. doi: 10.1128/jb.171.5.2803-2810.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., DeRosier D. J. Bacterial flagellar structure and function. Can J Microbiol. 1988 Apr;34(4):442–451. doi: 10.1139/m88-077. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Ornston M. K. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J Mol Biol. 1977 May 5;112(1):1–30. doi: 10.1016/s0022-2836(77)80153-8. [DOI] [PubMed] [Google Scholar]
- Ohta N., Mullin D. A., Tarleton J., Ely B., Newton A. Identification, distribution, and sequence analysis of new insertion elements in Caulobacter crescentus. J Bacteriol. 1990 Jan;172(1):236–242. doi: 10.1128/jb.172.1.236-242.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osley M. A., Newton A. Regulation of cell cycle events in asymmetrically dividing cells: functions required for DNA initiation and chain elongation in Caulobacter crescentus. J Bacteriol. 1978 Jul;135(1):10–17. doi: 10.1128/jb.135.1.10-17.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter J. S. Selection for nonbuoyant morphological mutants of Caulobacter crescentus. J Bacteriol. 1978 Sep;135(3):1141–1145. doi: 10.1128/jb.135.3.1141-1145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOVEPOINDEXTER J. L., COHEN-BAZIRE G. THE FINE STRUCTURE OF STALKED BACTERIA BELONGING TO THE FAMILY CAULOBACTERACEAE. J Cell Biol. 1964 Dec;23:587–607. doi: 10.1083/jcb.23.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. M., Stanier R. Y. The development of cellular stalks in bacteria. J Cell Biol. 1966 Mar;28(3):423–436. doi: 10.1083/jcb.28.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenlein P. V., Ely B. Characterization of strains containing mutations in the contiguous flaF, flbT, or flbA-flaG transcription unit and identification of a novel fla phenotype in Caulobacter crescentus. J Bacteriol. 1989 Mar;171(3):1554–1561. doi: 10.1128/jb.171.3.1554-1561.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenlein P. V., Gallman L. S., Ely B. Organization of the flaFG gene cluster and identification of two additional genes involved in flagellum biogenesis in Caulobacter crescentus. J Bacteriol. 1989 Mar;171(3):1544–1553. doi: 10.1128/jb.171.3.1544-1553.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. Generation of polarity during Caulobacter cell differentiation. Annu Rev Cell Biol. 1985;1:173–207. doi: 10.1146/annurev.cb.01.110185.001133. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Maizel J. V., Jr Synthesis and structure of Caulobacter crescentus flagella. J Bacteriol. 1973 Jan;113(1):478–485. doi: 10.1128/jb.113.1.478-485.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Agabian N. Caulobacter crescentus pili: analysis of production during development. Dev Biol. 1982 Jan;89(1):237–247. doi: 10.1016/0012-1606(82)90310-4. [DOI] [PubMed] [Google Scholar]
- Smit J., Agabian N. Cell surface patterning and morphogenesis: biogenesis of a periodic surface array during Caulobacter development. J Cell Biol. 1982 Oct;95(1):41–49. doi: 10.1083/jcb.95.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley J. T., Jordan T. L. Crossbands of Caulobacter crescentus stalks serve as indicators of cell age. Nature. 1973 Nov 16;246(5429):155–156. doi: 10.1038/246155a0. [DOI] [PubMed] [Google Scholar]
- Stallmeyer M. J., Hahnenberger K. M., Sosinsky G. E., Shapiro L., DeRosier D. J. Image reconstruction of the flagellar basal body of Caulobacter crescentus. J Mol Biol. 1989 Feb 5;205(3):511–518. doi: 10.1016/0022-2836(89)90222-2. [DOI] [PubMed] [Google Scholar]