Abstract
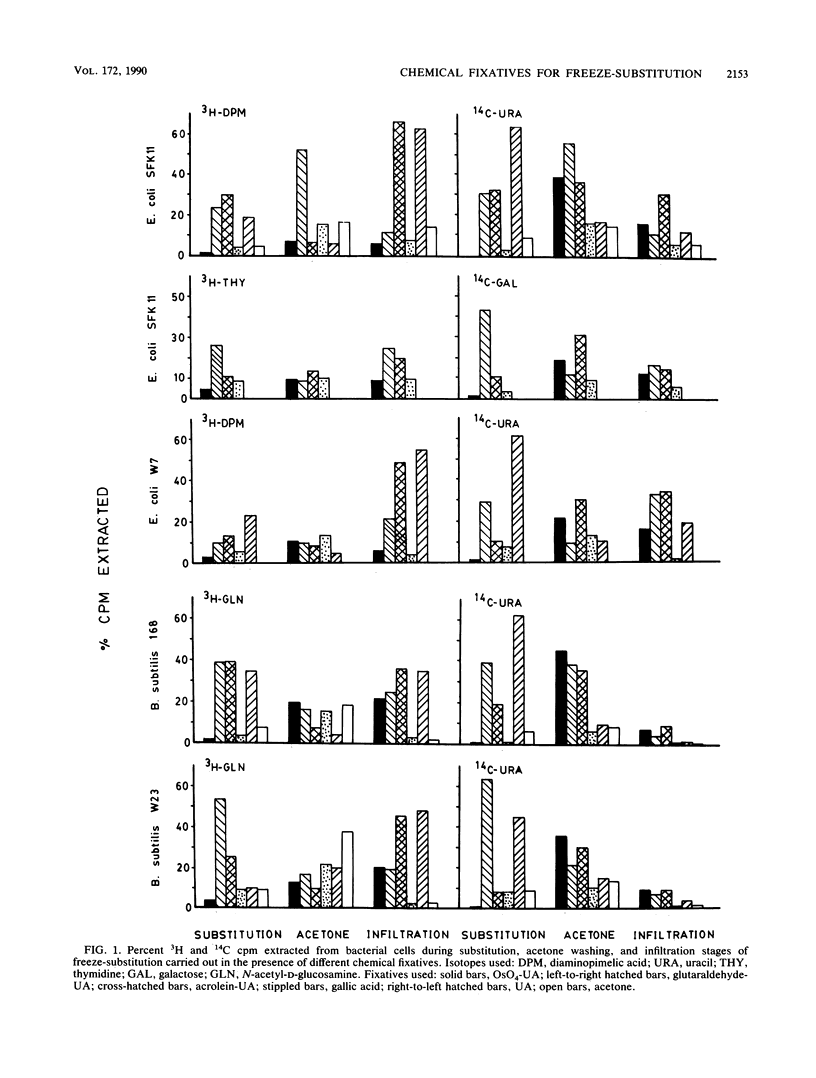
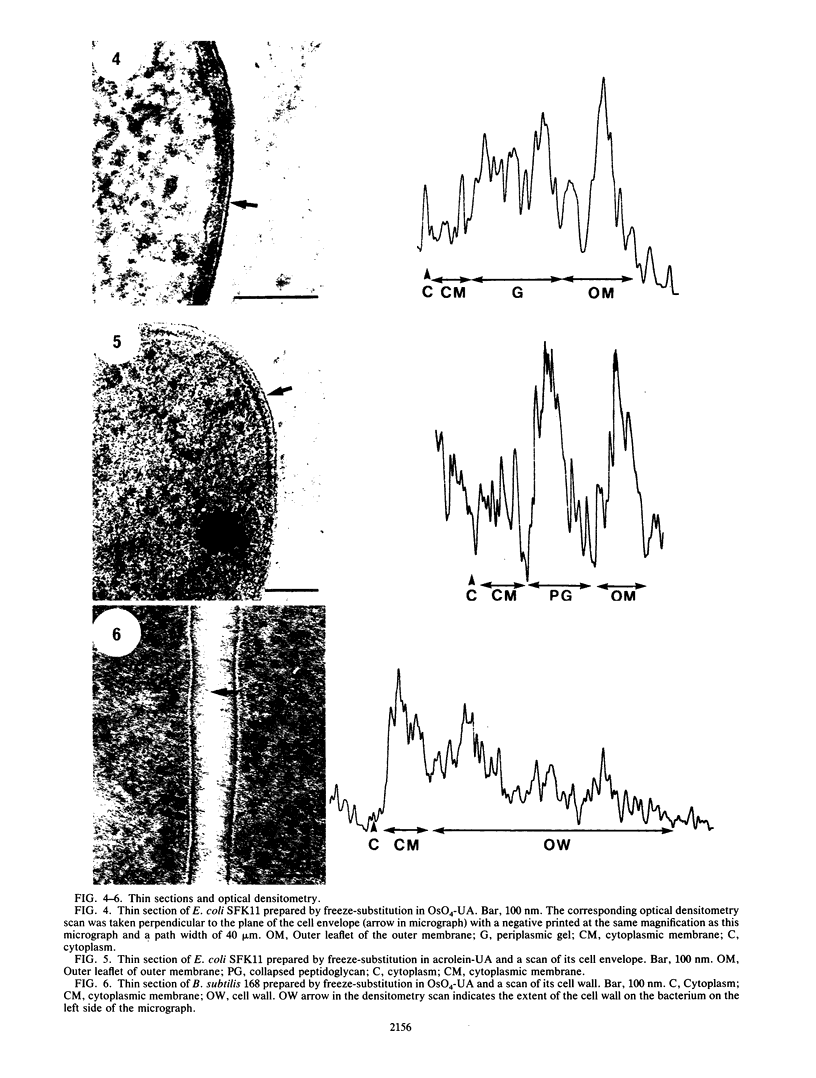
Five chemical fixatives were evaluated for their ability to accurately preserve bacterial ultrastructure during freeze-substitution of select Escherichia coli and Bacillus subtilis strains. Radioisotopes were specifically incorporated into the peptidoglycan, lipopolysaccharide, and nucleic acids of E. coli SFK11 and W7 and into the peptidoglycan and RNA of B. subtilis 168 and W23. The ease of extraction of radiolabels, as assessed by liquid scintillation counting during all stages of processing for freeze-substitution, was used as an indicator of cell structural integrity and retention of cellular chemical composition. Subsequent visual examination by electron microscopy was used to confirm ultrastructural conformation. The fixatives used were: 2% (wt/vol) osmium tetroxide and 2% (wt/vol) uranyl acetate; 2% (vol/vol) glutaraldehyde and 2% (wt/vol) uranyl acetate; 2% (vol/vol) acrolein and 2% (wt/vol) uranyl acetate; 2% (wt/vol) gallic acid; and 2% (wt/vol) uranyl acetate. All fixatives were prepared in a substitution solvent of anhydrous acetone. Extraction of cellular constituents depended on the chemical fixative used. A combination of 2% osmium tetroxide-2% uranyl acetate or 2% gallic acid alone resulted in optimum fixation as ascertained by least extraction of radiolabels. In both gram-positive and gram-negative organisms, high levels of radiolabel were detected in the processing fluids in which 2% acrolein-2% uranyl acetate, 2% glutaraldehyde-2% uranyl acetate, or 2% uranyl acetate alone were used as fixatives. Ultrastructural variations were observed in cells freeze-substituted in the presence of different chemical fixatives. We recommend the use of osmium tetroxide and uranyl acetate in acetone for routine freeze-substitution of eubacteria, while gallic acid is recommended for use when microanalytical processing necessitates the omission of osmium.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amako K., Meno Y., Takade A. Fine structures of the capsules of Klebsiella pneumoniae and Escherichia coli K1. J Bacteriol. 1988 Oct;170(10):4960–4962. doi: 10.1128/jb.170.10.4960-4962.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amako K., Murata K., Umeda A. Structure of the envelope of Escherichia coli observed by the rapid-freezing and substitution fixation method. Microbiol Immunol. 1983;27(1):95–99. doi: 10.1111/j.1348-0421.1983.tb03571.x. [DOI] [PubMed] [Google Scholar]
- Amako K., Okada K., Miake S. Evidence for the presence of a capsule in Vibrio vulnificus. J Gen Microbiol. 1984 Oct;130(10):2741–2743. doi: 10.1099/00221287-130-10-2741. [DOI] [PubMed] [Google Scholar]
- Amako K., Takade A. The fine structure of Bacillus subtilis revealed by the rapid-freezing and substitution-fixation method. J Electron Microsc (Tokyo) 1985;34(1):13–17. [PubMed] [Google Scholar]
- Beveridge T. J. The bacterial surface: general considerations towards design and function. Can J Microbiol. 1988 Apr;34(4):363–372. doi: 10.1139/m88-067. [DOI] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Septum formation in Escherichia coli: characterization of septal structure and the effects of antibiotics on cell division. J Bacteriol. 1974 Jul;119(1):303–324. doi: 10.1128/jb.119.1.303-324.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee J., van der Merwe C. F. Extraction of carbon 14-labeled compounds from plant tissue during processing for electron microscopy. J Electron Microsc Tech. 1989 Feb;11(2):155–160. doi: 10.1002/jemt.1060110210. [DOI] [PubMed] [Google Scholar]
- Cope G. H., Williams M. A. Quantitative studies on the preservation of choline and ethanolamine phosphatides during tissue preparation for electron microscopy. I. Glutaraldehyde, osmium tetroxide, Araldite methods. J Microsc. 1969;90(1):31–46. doi: 10.1111/j.1365-2818.1969.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Cope G. H., Williams M. A. Quantitative studies on the preservation of choline and ethanolamine phosphatides during tissue preparation for electron microscopy. II. Other preparative methods. J Microsc. 1969;90(1):47–60. doi: 10.1111/j.1365-2818.1969.tb00693.x. [DOI] [PubMed] [Google Scholar]
- Graham L. L., Beveridge T. J. Evaluation of freeze-substitution and conventional embedding protocols for routine electron microscopic processing of eubacteria. J Bacteriol. 1990 Apr;172(4):2141–2149. doi: 10.1128/jb.172.4.2141-2149.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey D. M. Freeze-substitution. J Microsc. 1982 Aug;127(Pt 2):209–221. doi: 10.1111/j.1365-2818.1982.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Hobot J. A., Carlemalm E., Villiger W., Kellenberger E. Periplasmic gel: new concept resulting from the reinvestigation of bacterial cell envelope ultrastructure by new methods. J Bacteriol. 1984 Oct;160(1):143–152. doi: 10.1128/jb.160.1.143-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobot J. A., Villiger W., Escaig J., Maeder M., Ryter A., Kellenberger E. Shape and fine structure of nucleoids observed on sections of ultrarapidly frozen and cryosubstituted bacteria. J Bacteriol. 1985 Jun;162(3):960–971. doi: 10.1128/jb.162.3.960-971.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Doyle R. J., Streips U. N., Langemeier S. O. Transport and incorporation of N-acetyl-D-glucosamine in Bacillus subtilis. J Bacteriol. 1982 Apr;150(1):8–15. doi: 10.1128/jb.150.1.8-15.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata F., Suzuki S., Tsuyama S., Suganuma T., Imada M., Furihata C. Application of rapid freezing followed by freeze-substitution acrolein fixation for cytochemical studies of the rat stomach. Histochem J. 1985 Sep;17(9):967–980. doi: 10.1007/BF01417946. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T., Sousa J. C. Ultrastructure of the cell wall and cytoplasmic membrane of gram-negative bacteria with different fixation techniques. J Bacteriol. 1973 Feb;113(2):953–962. doi: 10.1128/jb.113.2.953-962.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda A., Ueki Y., Amako K. Structure of the Staphylococcus aureus cell wall determined by the freeze-substitution method. J Bacteriol. 1987 Jun;169(6):2482–2487. doi: 10.1128/jb.169.6.2482-2487.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibull C., Christiansson A., Carlemalm E. Extraction of membrane lipids during fixation, dehydration and embedding of Acholeplasma laidlawii-cells for electron microscopy. J Microsc. 1983 Feb;129(Pt 2):201–207. doi: 10.1111/j.1365-2818.1983.tb04174.x. [DOI] [PubMed] [Google Scholar]
- Weibull C., Villiger W., Carlemalm E. Extraction of lipids during freeze-substitution of Acholeplasma laidlawii-cells for electron microscopy. J Microsc. 1984 May;134(Pt 2):213–216. doi: 10.1111/j.1365-2818.1984.tb02513.x. [DOI] [PubMed] [Google Scholar]







