Abstract
Connexin 43 (Cx43) nonjunctional or “unapposed” hemichannels can open under physiological or pathological conditions. We characterize hemichannels comprised of Cx43 or Cx43-EGFP (Cx43 with enhanced GFP fused to the C terminus) expressed in HeLa cells. Channel opening was induced at potentials greater than +60 mV. Open probability appeared to be very low. No comparable opening was detected in the parental, nontransfected HeLa cells. Conductance of fully open single hemichannels was ≈220 pS, which is approximately double that of Cx43 cell–cell channels. Cx43 hemichannels exhibited two types of gating: fast transitions (<1 ms) between the fully open state and a substate of ≈75 pS and slow transitions (>5 ms) between either open state and the fully closed state. Cx43-EGFP hemichannels exhibited only slow transitions (>5 ms) between closed and fully open states. These properties resemble those of the corresponding Cx43 and Cx43-EGFP cell–cell channels. Cx43 with EGFP on the N terminus (EGFP-Cx43) inserted into the surface and formed plaques but did not form hemichannels or cell–cell channels. Hemichannel blockers, 18β-glycyrrhetinic acid or La3+, blocked depolarization-induced currents. Uptake of ethidium bromide (i) was faster in Cx43 and Cx43-EGFP than parental and EGFP-Cx43 cells, (ii) was directly correlated with Cx43-EGFP expression, (iii) was reduced by hemichannel blockers, and (iv) occurred at the same low rate in EGFP-Cx43 and parental cells. Although hemichannel opening was not detected electrophysiologically at the resting potential, infrequent or brief opening could account for ethidium bromide uptake. Opening of Cx43 hemichannels may mediate normal signaling or be deleterious.
Keywords: gap junction, connexon, dye uptake, permeation
Connexins belong to a protein family consisting of ≈20 members and are widely distributed in mammalian tissues. Connexins are assembled into hexamers and inserted into the plasma membrane where they dock, one to one, with hexamers in an apposed membrane to form gap junction channels. Thus, in a formed junction each hexamer is a hemichannel (or connexon). Surface expression of nonjunctional or unapposed hemichannels has been demonstrated in several cell lines and primary cultures by electron microscopic, biochemical, and/or electrophysiological methods (1–6). Recent evidence indicates that new channels are added to the periphery of gap junctions and that older channels are internalized in the central region of the plaque (7, 8). Because open hemichannels were expected to have a high conductance and permeability, it was long thought that they remained closed until docking with a hemichannel in an apposed membrane (e.g., ref. 9). Indeed, direct measurement of the first gap junction channel forming between a pair of cells indicated that nonjunctional conductance did not change during the formation period, thus indicating that the two hemichannels were indeed closed before cell–cell channel formation (10, 11). More recently, connexin 43 (Cx43) hemichannels have been implicated in diverse roles in cell physiology and pathology including volume regulation (12), efflux of NAD+ and ATP (13–16), limitation of apoptosis in osteoblastic cells (17), and acceleration of cell death during metabolic inhibition (3, 18, 19). However, the biophysical properties of Cx43 hemichannels are not yet described.
In a number of reports, the opening of hemichannels is enhanced by polarization to positive membrane potentials, reduction in extracellular calcium concentration, or both (4, 6, 20–23). Conversely, closure of hemichannels is induced by low extracellular pH (24), gap junction channel blockers such as glycyrrhizin agents (18α- and 18β-glycyrrhetinic acid) (18), trivalent cations (La3+ or Gd3+) (3, 16, 18), and some chloride channel blockers [flufenamic acid, niflumic acid, and 5-nitro-2(3-phenylpropylamino)benzoic acid] (16, 25). Hemichannel currents in recombinant systems expressing Cx30, Cx45, Cx46, or Cx50 have been characterized at the single-channel level (5, 6, 24, 26, 27).
In the present study we used HeLa cells transfected with Cx43 or Cx43-enhanced GFP (EGFP) to characterize the electrophysiological properties of single Cx43 and Cx43-EGFP hemichannels. We found that their unitary open-state conductance and voltage-gating modalities were consistent with the properties in the corresponding cell–cell channels in which two hemichannels are in series. Moreover, we observed cell uptake of ethidium bromide (EtdBr) ascribable to infrequent opening of hemichannels in the absence of overt stimulation.
Materials and Methods
Cell Cultures. Experiments were performed on HeLa cells (ATCC no. CCL-2, the parental cells) or HeLa cells transfected with cDNAs encoding rat Cx43 or rat Cx43 with EGFP attached to the C or N terminus (Cx43-EGFP or EGFP-Cx43, respectively) and were maintained in culture as described (28). The cDNA constructs encoding Cx43-EGFP or EGFP-Cx43 were made as described (28, 29). Cx43-EGFP and EGFP-Cx43 cells could be recognized by their fluorescence emission at 530 nm. For electrophysiology or fluorescence studies, cells were seeded onto sterile glass-bottom dishes (MaTek, Ashland, MA) or onto coverslips placed in culture dishes at 103 cells per cm2 16–24 h before each experiment. Mixed parental and Cx43-EGFP cultures were made with a 2:1 ratio of parental/Cx43-EGFP cells.
Electrophysiological Measurements. For simultaneous electrophysiological and fluorescence recording, cells were grown on coverslips and then transferred to an experimental chamber mounted on the stage of an inverted Olympus (Melville, NY) IX-70 microscope. The chamber was perfused with modified Krebs–Ringer solution containing 140 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 10 mM Hepes, pH 7.4. The pipette solution contained 130 mM KCl, 10 mM sodium aspartate, 0.26 mM CaCl2, 2 mM EGTA, 5 mM tetraethylammonium-Cl, 1 mM MgCl2, 3 mM MgATP, and 5 mM Hepes at pH 7.2. Whole-cell currents were recorded as described (28). For data acquisition and analysis, we used an MIO-16X A/D converter (National Instruments, Austin, TX) and in-house software by E. B. Trexler and V. K. Verselis. Shown are means ± SD.
EtdBr Uptake and EGFP Fluorescence. For time-lapse recording of EtdBr uptake, we used a microincubator adapted to fit on the stage of an Olympus IX-70 inverted microscope and a fluorescence imaging system with a ×60 oil immersion objective. Temperature was maintained at 37°C, and the chamber was perfused with humidified air. Cells were bathed in Krebs–Ringer solution with 10 μM EtdBr, and images were acquired with an OlymPix 2000 cooled digital camera (Olympus) and ultraview software (Perkin–Elmer). EGFP fluorescence was monitored as described (30, 31).
Results
Positive Membrane Potentials Activate Currents with Conductance and Voltage Gating Appropriate for Cx43 Hemichannels. HeLa cells expressing Cx43 or Cx43-EGFP exhibited no readily detectable channel openings at their resting potentials or for polarizations of ±30 mV. Resting potentials and conductances, determined for cells not in contact with other cells, were not significantly different for the transfectants and parental cells (P > 0.05 by ANOVA with Kolmogorov–Smirnov correction; Table 1).
Table 1. Resting potentials and conductances of parental and transfected cells.
| Cell type | Resting potential, mV | Conductance, pS |
|---|---|---|
| Parental (n = 12) | -23 ± 11 | 166 ± 63 |
| Cx43 (n = 14) | -14 ± 4 | 261 ± 66 |
| Cx43-EGFP (n = 17) | -15 ± 8 | 208 ± 95 |
| EGFP-Cx43 (n = 4) | -19 ± 11 | 169 ± 34 |
Resting conductances were comparable to that of a single hemichannel as shown below, such that single openings lasting >1 ms should have been detected. If there were many openings too brief to be recorded (filtering at 1,000–3,000 Hz), they still did not produce much net charge transfer compared with that of a single channel continuously open. Moreover, hemichannel blockers did not decrease cell conductance (see below).
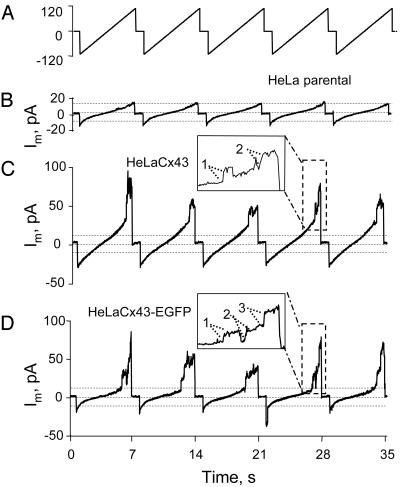
During application of slow voltage ramps (6 s with 1 s between ramps) from –110 to +110 mV, pronounced increases in current were apparent in Cx43 and Cx43-EGFP cells at large positive potentials (Fig. 1 C and D, see expanded trace at ≈28 s). The increases exhibited frequent steps corresponding to a conductance of ≈220 pS (assuming a reversal potential of 0 mV; see Fig. 1 C and D Insets, which show expanded traces at ≈28 s; well resolved steps are indicated by dotted lines). Channels opened by positivity were closed by the time the next ramp began with a transition to –110 mV (except for the ramp in shown in Fig. 1D beginning at 22 s). In parental cells there were much smaller increases in currents at large negative and at large positive potentials (Fig. 1B). Cx43 and Cx43-EGFP cells also showed small increases in current at large negative potentials comparable to those in parental cells.
Fig. 1.
Positive voltages induce hemichannel currents in HeLa cells expressing Cx43 and Cx43-EGFP but not in parental cells. (A) Voltage ramps from –110 to +110mV, 6sin duration separated by 1 s, were applied; the zero potential was the resting potential. (B–D) Currents of parental, Cx43, and Cx43-EGFP cells, respectively. Large increases in current were evoked at voltages greater than +60 mV in Cx43 and Cx43-EGFP cells (C and D), but only small increases were evoked in parental cells (B). All cells show small increases in conductance at large negative potentials. Expanded records are shown for the ramps in B and C ending at 28 s. Presumptive single-channel openings are indicated by dotted lines. Open channels closed by the beginning of the next ramp except for the ramp ending at 21 s (D). Records in this figure are representative of 15 cells of each type.
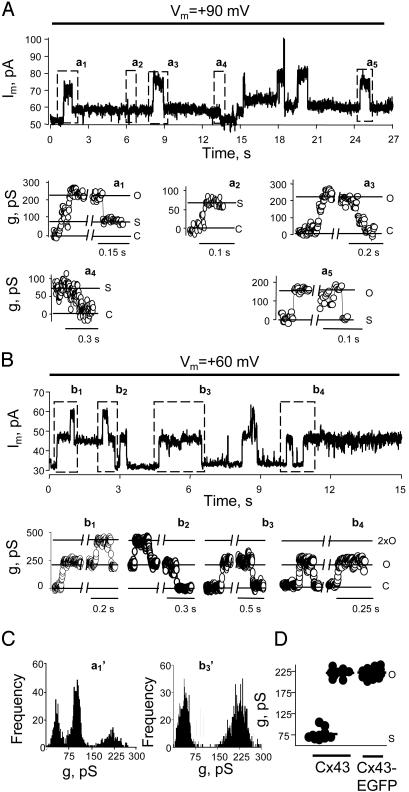
To examine single-channel activity in greater detail, we applied steady holding potentials to connexin-expressing cells. Held at +90 mV, a Cx43 cell exhibited two conductance states corresponding to a fully open state of 220 ± 10 pS and a substate of 77 ± 13 pS (assuming a reversal potential of 0 mV; Fig. 2 A Upper and D, n = 10 cells, several measurements per cell). Representative all-points histograms are shown for a1 and b3 in Fig. 2C. The transitions between closed and fully open states had a slow time course (>5 ms) (Fig. 2 A, a1, first transition, and a3, both transitions). Slow transitions also occurred between the fully closed state and the substate (Fig. 2 A, a2 and a4). Additionally, there were fast (<1 ms) transitions of ≈143 pS between the fully open state and the substate (Fig. 2 A, a1, second transition, and a5, both transitions). Comparable but smaller transitions and conductances are observed for Cx43 cell–cell channels; there are slow transitions between the closed and the fully open states (≈110 pS) and between the closed state and a substate of ≈30 pS, and there are fast transitions between the fully open state and the substate (28). The fully open conductance of the hemichannel is approximately twice that of the cell–cell channel, as predicted from series arrangement. (Access resistance for these channels, which are relatively long compared with their diameter, would make <5% difference in the ratio of conductances of the open channels and hemichannels; see Fig. 6 and Calculation of Relative Conductances of Hemichannels and Cell–Cell Channels, which are published as supporting information on the PNAS web site, www.pnas.org.) The observed hemichannel subconductance of ≈75 pS is more than twice the conductance of the substate of the cell–cell channel. The conductance of the hemichannel substate should be only slightly larger than the conductance of the substate of the cell–cell channel because the latter has an open hemichannel in series with the hemichannel in the substate (Fig. 6).
Fig. 2.
Unitary conductance and voltage gating of Cx43 and Cx43-EGFP hemichannels. (A Upper) Single-channel events in a Cx43 cell at a holding Vm of +90 mV. Two channels were fully open just after 18 s. (Lower) Boxed regions in Upper are shown as conductances on an expanded time scale (reversal potential assumed to be 0; leakage current = 52 pA; open circles indicate 2-ms time points). a1, A single channel opened at 1 s to a conductance of ≈220 pS and then closed to a substate of ≈75 pS. The opening was slow (>10 ms), but the closure to the substate was fast and not resolved. a2, Slow opening to the substate. a3, Slow opening and closing of a second channel superimposed on the substate of the first channel (redefined as zero current here). a4, Slow closing of the substate of the first channel. a5, Fast opening and closing of one channel superimposed on the substate of both channels. O, open state; S, substate; C, closed state. (B) Single-channel activity in a Cx43-EGFP cell; a maximum of two channels were open simultaneously. The display is as described for A.b1–b4, Records with higher time resolution showing only slow transitions between open and closed states. (C) All-points histograms from a1 and b3 showing three peaks for Cx43 and two for Cx43-EGFP. (D) Conductances of substate and main state; each point is the mean of several measurements from a different cell. The main state had the same conductance for Cx43 (220 ± 11 pS; n = 10) and Cx43-EGFP (223 ± 9 pS; n = 15). The conductance of the Cx43 substate was 77 ± 13 pS (n = 10).
Cx43-EGFP cell–cell channels have the same fully open unitary conductance as Cx43 channels, but Cx43-EGFP cell–cell channels do not show a substate and fast voltage gating (28). Similar to their cognate cell–cell channels, Cx43-EGFP hemichannels exhibited only slow transitions (>5 ms) between the fully open and the closed states (Fig. 2 B and C). The unitary conductance of the open state was 223 ± 9 pS (n = 15, Fig. 2D). No fast transitions between fully open and substates were seen in 25 recordings 3–10 min in duration.
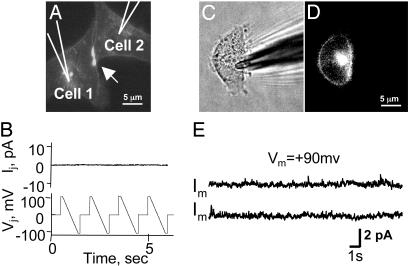
Cx43 with EGFP on the N Terminus (EGFP-Cx43) Is Transported to the Cell Surface and Forms Plaques but No Functional Hemichannels or Cell–Cell Channels. Transfection with connexin cDNAs can induce expression of other genes (32), and Cx43 has binding partners that associate with it (e.g., ZO-1 and caveolin-1) (33, 34). Thus, we were concerned that expression of Cx43 could induce surface expression of other channels that were responsible for the currents that we were identifying as hemichannel-mediated. To explore this question we used EGFP-Cx43-expressing cells. These cells formed junctional plaques (Fig. 3A, arrow), but there were no transjunctional currents when +100- to –100-mV voltage ramps were applied to one cell (Fig. 3B, n = 10 pairs). Although EGFP-Cx43 cells exhibited EGFP fluorescence at the membrane surface (Fig. 3 C and D), no hemichannel currents were recorded from them (Fig. 3E, n = 15 cells). Furthermore, there was no voltage-dependent current in addition to those detected in parental cells. Because the cytoplasmic C-terminal domain in EGFP-Cx43 is not directly modified and this domain in Cx43 is implicated in binding to other proteins, it is unlikely that depolarization-induced currents in Cx43 and Cx43-EGFP cells result from connexin-mediated transport of other proteins to the cell surface, and we conclude that the currents indeed are generated by functional hemichannels.
Fig. 3.
EGFP-Cx43 in HeLa cells reaches the cell surface and forms plaques but no functional hemichannels or channels. (A) A pair of EGFP-Cx43 cells forms a fluorescent plaque typical of a gap junction (arrow). (B) Application of voltage ramps (Lower) to cell 1 did not induce junctional currents in the other cell (Upper). (C) Phase. (D) Fluorescence of an isolated EGFP-Cx43 cell shows expression at the surface membrane. (E) Two records of polarization to +90 mV show no Cx43-like single-channel activity (an ≈20-pA step would be seen after opening of a 220-pS Cx43 channel; see Fig. 2).
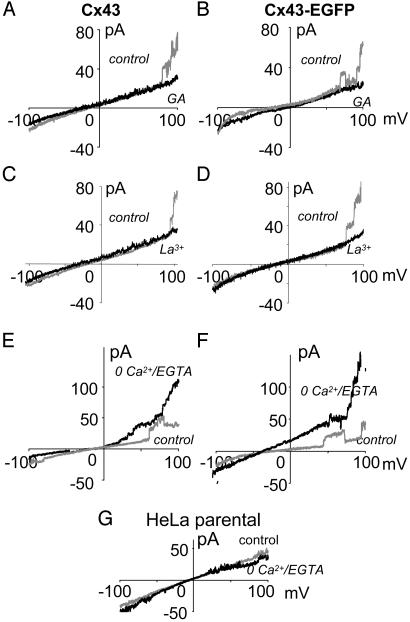
Depolarization-Induced Hemichannel Currents Have the Right Pharmacology. Hemichannel currents induced by positive potentials in Cx43 and Cx43-EGFP cells were completely blocked by application of the gap junction blocker, 18β-glycyrrhetinic acid (35 μM; Fig. 4 A and B). Similarly, La3+ (100 μM), which blocks dye uptake mediated by hemichannels, abolished these currents (Fig. 4 C and D). Thus, for these blockers, the observed hemichannel activity was affected as predicted.
Fig. 4.
Hemichannel opening is blocked by gap junction blockers and potentiated by zero Ca2+/EGTA. (B, D, and F) Cx43 cells. (A, C, and E) Cx43-EGFP cells. –/+ voltage ramps were applied as described for Fig. 1. (A and B) α-Glycyrrhetinic acid (GA, 35 μM) blocked channel opening at positive potentials with little effect on resting conductance. (C and D) La3+ also blocked hemichannel currents with little effect on resting conductance. (E and F) Zero Ca2+/EGTA (2 mM) increased opening at positive potentials, and occasionally channels remained open between ramps (F), but there was little effect on resting conductance. (G) Zero Ca2+/EGTA had little effect on parental cells over the entire voltage range.
In a number of cell types, zero Ca2+ induces openings of surface hemichannels (see the Introduction), and we expected that positive potentials would induce more hemichannel activity in Cx43 and Cx43-EGFP cells in zero Ca2+ plus 2 mM EGTA. Under control conditions (1.8 mM extracellular Ca2+), Cx43 and Cx43-EGFP cells did not show more than one to three single hemichannel openings during the positive phase of voltage ramps (Fig. 4 E and F). After perfusion with zero Ca2+ plus 2 mM EGTA, many more hemichannel openings were observed. In most if not all cells (n = 6), basal conductance was increased for a few ramps, indicating that one or two hemichannels remained open between ramps and during the next negative phase. No significant differences were observed in parental cells in the zero Ca2+/EGTA solution (Fig. 4G).
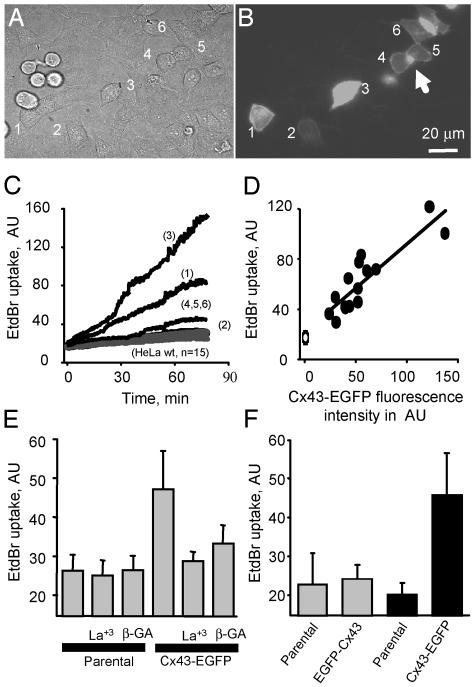
EtdBr Uptake by Cx43-EGFP Cells Is Mediated by Hemichannels. Dye uptake has been used as an indicator of hemichannel opening in a number of studies (see the Introduction). To investigate the possibility of hemichannel opening under normal conditions further, we evaluated the uptake of EtdBr from the extracellular milieu in control solution. Cocultures of parental and Cx43-EGFP cells (Fig. 5 A and B; see also Fig. 7, which is published as supporting information on the PNAS web site) were incubated in 10 μM EtdBr. Cx43-EGFP cells showed significantly more EtdBr uptake than parental cells (Figs. 5C and 7; see also Movie 1, which is published as supporting information on the PNAS web site). Fluorescence increased fairly linearly over periods of 75–180 min and gave no indication of saturation; thus the time constant of dye equilibration is long compared with these periods. When different cells were compared, the amount of EtdBr uptake was directly proportional to the level of Cx43-EGFP fluorescence (Fig. 5D, the point on the y intercept is mean uptake by parental cells). These data suggest that cells with more Cx43-EGFP have more functional hemichannels at their cell surface. (Fluorescence was measured as total fluorescence minus background and minus fluorescence of gap junction plaques and obvious cytoplasmic concentrations of Cx43-EGFP.) In addition, the hemichannel blockers La3+ (0.1 mM) and β-glycyrrhetinic acid (35 μM) reduced EtdBr uptake in Cx43-EGFP cells but not in parental cells (Fig. 5E). However, when a high concentration of EtdBr (100–500 μM) was applied, no differences in dye uptake were observed between parental and Cx43-EGFP cells, suggesting toxicity or the existence of low-affinity uptake mechanisms independent of Cx43 hemichannels; this dye uptake was not affected by hemichannel blockers (data not shown).
Fig. 5.
EtdBr uptake by Cx43-EGFP cells in standard culture conditions is mediated by opening of hemichannels. (A) Phase contrast of a mixed culture of parental and Cx43-EGFP cells seeded 16 h before dye uptake assay. (B) Cx43-EGFP fluorescence of the field shown in A; six cells expressing different levels of EGFP are numbered 1–6. Cells 4–6 are in contact and show fluorescent gap junction plaques between them (arrows). The rounded-up cells on the left are dividing parental cells; flattened interphase parental cells are also present. (C) Cells were incubated in 10 μM EtdBr, and dye uptake was measured every 45 s as fluorescence emission of EtdBr binding to DNA [518 nm, arbitrary units (AU) of intensity]. Uptake was greatest in cell 3, which exhibited the most EGFP fluorescence, and less for the other cells. Uptake for parental cells (n = 15) was less than for any of the Cx43-EGFP cells. (D) Plot of EtdBr fluorescence (at 60 min) as a function of Cx43-EGFP fluorescence for 13 cells and for mean uptake by parental cells (n = 15). Uptake was linearly related to EGFP fluorescence (r = 0.79 for Cx43-EGFP cells). (E) Gap junction blockers La3+ (0.1 mM) and 18β-glycyrrhetinic acid (GA, 35 μM) reduced EtdBr uptake by Cx43-EGFP cells (P < 0.05 for parental vs. Cx43-EGFP, other pairings not significant by t test) but did not affect uptake by parental cells. (F) Dye uptake after a 75-min incubation with 10 μM EtdBr in mixed cultures of parental cells with EGFP-Cx43 cells (gray bars) or mixed cultures of parental with Cx43-EGFP cells (black bars). EtdBr uptake was low and not significantly different in EGFP-Cx43 and parental cells (P > 0.3, n = 20 for each cell type); EtdBr uptake was greater in Cx43-EGFP than in parental cells (P < 0.05, n = 20 for each cell type).
To demonstrate further that EtdBr uptake is mediated by hemichannels, EGFP-Cx43 and Cx43-EGFP cells were compared with parental cells with respect to dye uptake. EGFP-Cx43 and parental cells showed similarly low dye uptake, whereas Cx43-EGFP cells showed significantly greater dye uptake (Fig. 5F, P < 0.05, n = 20). Because EGFP-Cx43 does not form open hemichannels but is otherwise similar to Cx43-EGFP, this result supports mediation of dye uptake by open hemichannels.
Discussion
We demonstrate here the existence of Cx43 single hemichannel currents in transfected mammalian HeLa cells. The evidence is that (i) depolarization induces channel activity in Cx43 and Cx43-EGFP cells but not in parental cells; (ii) the single-channel conductance is twice that of Cx43 and Cx43-EGFP cell–cell channels, as predicted from series arrangement of two hemichannels in a cell–cell channel; (iii) slow- and fast-gating transitions are seen in Cx43 cell–cell channels and Cx43 hemichannels, whereas only slow transitions are seen in Cx43-EGFP cell–cell channels and hemichannels; (iv) EGFP-Cx43, which forms apparent junctional plaques but no functional cell–cell channels, does not form voltage-activated hemichannels; (ν) the hemichannel currents are abolished by gap junction and hemichannel blockers; and (vi) as determined in other systems and for other connexins, depolarization and zero Ca2+/EGTA enhance opening of Cx43 hemichannels.
Although the unitary conductance of the fully open state and fast- and slow-gating transitions of Cx43 hemichannels are consistent with those of the cell–cell channels, the substate conductance is greater than expected (as was also observed for other connexin hemichannels; see ref. 35). The explanation for this discrepancy may lie in the detailed structure of the fast gate and how its configuration differs in hemichannels and cell–cell channels. Hemichannels also differ from cell–cell channels in their low open probability, at least if the membrane-associated fluorescence of Cx43-EGFP is entirely due to hemichannels in the surface membrane. Antibody staining of an extracellular epitope establishes surface location of the hemichannels in cultured astrocytes (2). This apparent difference remains even when taking into account the low open probability (≈0.1) of cell–cell channels in gap junction plaques. The (unknown) reason for the near-zero open probability of cell–cell channels in plaques of a few hundred channels (30) may account for the low open probability of hemichannels; hemichannels, similar to cell–cell channels, may have to be in relatively large aggregates before even one of them opens (36).
As noted, the voltage-gating properties observed in the single Cx43 and Cx43-EGFP hemichannel currents were similar to those of their respective cell–cell channels (28). Cx43 cell–cell channels have two distinct gating mechanisms: a fast and a slow gate. Functional separation of these gates is supported by molecular studies. Attaching EGFP or aequorin to the C terminus of Cx43 blocks fast gating with little or no effect on slow gating (28, 37). Truncation of the C terminus can block fast gating while leaving slow gating intact (38). Mutations in the N terminus also affect fast gating and can reverse its polarity (39–41).
The failure of EGFP-Cx43 to show hemichannel activity is evidence against the transport of another channel-forming molecule to the cell surface by Cx43. Because the C terminus of EGFP-Cx43 is not directly altered in the chimera, any trafficking function of Cx43 is likely to be unimpaired; moreover, Cx43-EGFP would be more likely to have its trafficking functions compromised.
Another treatment that induces opening of hemichannels, at least in some systems, is bathing in low Ca2+ solution (and more severe depletion by the addition of a Ca2+ chelator may be required). Our electrophysiological data showed that opening of Cx43 and Cx43-EGFP hemichannels was increased modestly at positive potentials in zero Ca2+ or zero Ca2+/EGTA, and occasional openings continued through the negative phase of the succeeding ramp. A low probability of hemichannel opening at low Ca2+ might explain the low percentage of cells showing dye uptake in low Ca2+ even though all cells express Cx43 (3, 17–19, 42).
Although Cx43-EGFP expression increased membrane permeability to EtdBr in normal conditions, we did not observe single-channel currents in most cells without polarization to positive potentials. We might have missed openings at the resting potential because of small amplitude, low frequency, and possibly brief duration. Furthermore, several lines of evidence indicate that EtdBr uptake by cells under these conditions was due to basal opening of Cx43 hemichannels: (i) uptake was linearly related to Cx43-EGFP expression and much greater in Cx43 and Cx43-EGFP cells than in parental cells and EGFP-Cx43 cells; (ii) uptake by Cx43 and Cx43-EGFP cells was reduced by gap junction blockers, but uptake by parental cells was not affected; and (iii) application of EtdBr did not induce hemichannel currents (data not shown). Our observations are in agreement with previous reports that suggest efflux of NAD+ through Cx43 hemichannels by passive diffusion under basal conditions (13). The time constant for a single channel passing a tracer into a cell the size of a HeLa cell would be ≈1–4 h (see Calculation of Time Constant of Equilibration of Dye Influx as a Function of Single Hemichannel Permeability and Number of Channels, which is published as supporting information on the PNAS web site). We saw no saturation of uptake in 1–3 h, indicating that the time constant was at least severalfold greater (Fig. 5C). The conductance near the resting potential was of the order of a single channel and not noticeably changed by gap junction blockers; thus only a small fraction of the resting conductance could have been due to brief opening of hemichannels. Thus, we do not account for EtdBr uptake by a measured hemichannel conductance, but neither is there a clear discrepancy. The effect of hemichannel blockers argues for uptake mediated by unresolved openings, but we cannot yet exclude uptake mediated without increase in conductance.
In summary, we demonstrate here the existence of functional, unapposed Cx43 hemichannels in mammalian cells and provide an electrophysiological characterization. Release of cytoplasmic molecules by regulated opening of hemichannels is likely to have a physiological role under some circumstances. Conversely, inappropriate opening of connexin hemichannels may increase metabolic stress and lead to cell death. The data provided here should facilitate future studies of the role of hemichannels formed of Cx43 and other connexins in cellular function and pathology.
Supplementary Material
Acknowledgments
We thank Angela Buskauskiene for outstanding technical assistance. This work was partially funded by National Institutes of Health Grants NS45837 (to M.V.L.B.) and NS36706 (to F.F.B.), a grant from the F. M. Kirby Foundation Program in Neuroprotection and Repair, and Fondo Nacional para el Desarrollo de Ciencia y Tecnología Grant 1030945 (to J.C.S.).
Abbreviations: Cx, connexin; EGFP, enhanced GFP; EtdBr, ethidium bromide.
References
- 1.Musil, L. S. & Goodenough, D. A. (1991) J. Cell Biol. 115, 1357–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofer, A. & Dermietzel, R. (1998) Glia 24, 141–154. [DOI] [PubMed] [Google Scholar]
- 3.John, S. A., Kondo, R., Wang, S. Y., Goldhaber, J. I. & Weiss. J. N. (1999) J. Biol. Chem. 274, 236–240. [DOI] [PubMed] [Google Scholar]
- 4.Li, H., Liu, T. F., Lazrak, A., Peracchia, C., Goldberg, G. S., Lampe, P. D. & Johnson, R. G. (1996) J. Cell Biol. 134, 1019–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valiunas, V. & Weingart, R. (2000) Pflügers Arch. 440, 366–379. [DOI] [PubMed] [Google Scholar]
- 6.Valiunas, V. (2002) J. Gen. Physiol. 119, 147–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaietta, G., Deerinck, T. J., Adams, S. R., Bouwer, J., Tour, O., Laird, D. W., Sosinsky, G. E., Tsien, R. Y. & Ellisman, M. H. (2002) Science 296, 503–507. [DOI] [PubMed] [Google Scholar]
- 8.Lauf, U., Giepmans, B. N., Lopez, P., Braconnot, S., Chen, S. C. & Falk, M. M. (2002) Proc. Natl. Acad. Sci. USA 99, 10446–10451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett, M. V. L., Barrio, L. C., Bargiello, T. A., Spray, D. C., Hertzberg, E. & Sáez, J. C. (1991) Neuron 6, 305–320. [DOI] [PubMed] [Google Scholar]
- 10.Bukauskas, F. F. & Weingart, R. (1994) Biophys. J. 67, 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukauskas, F. F., Elfgang, C., Willecke, K. & Weingart, R. (1995) Biophys. J. 68, 2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quist, A. P., Rhee, S. K., Lin, H. & Lal, R. (2000) J. Cell. Biol. 148, 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruzzone, S., Guida, L., Zocchi, E., Franco, L. & De Flora, A. (2001) FASEB J. 15, 10–12. [DOI] [PubMed] [Google Scholar]
- 14.Franco, L., Zocchi, E., Usai, C., Guida, L., Bruzzone, S., Costa, A. & De Flora, A. (2001) J. Biol. Chem. 276, 21642–21648. [DOI] [PubMed] [Google Scholar]
- 15.Cotrina, M. L., Lin, J. H., Alves-Rodrigues, A., Liu, S., Li, J., Azmi-Ghadimi, H., Kang, J., Naus, C. C. & Nedergaard, M. (1998) Proc. Natl. Acad. Sci. USA 95, 15735–15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stout, C. E., Costantin, J. L., Naus, C. C. & Charles, A. C. (2002) J. Biol. Chem. 277, 10482–10488. [DOI] [PubMed] [Google Scholar]
- 17.Plotkin, L. I., Manolagas, S. C. & Bellido, T. (2002) J. Biol. Chem. 277, 8648–8657. [DOI] [PubMed] [Google Scholar]
- 18.Contreras, J. E., Sanchez, H. A., Eugenin, E. A., Speidel, D., Theis, M., Willecke, K., Bukauskas, F. F., Bennett, M. V. L. & Sáez, J. C. (2002) Proc. Natl. Acad. Sci. USA 99, 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo, R. P., Wang, S. Y., John, S. A., Weiss, J. N. & Goldhaber, J. I. (2000) J. Mol. Cell. Cardiol. 32, 1859–1872. [DOI] [PubMed] [Google Scholar]
- 20.Paul, D. L., Ebihara, L., Takemoto, L. J., Swenson, K. I. & Goodenough, D. A. (1991) J. Cell Biol. 115, 1077–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeVries, S. H. & Schwartz, E. A. (1992) J. Physiol. (London) 445, 201–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebihara, L. & Steiner, E. (1993) J. Gen. Physiol. 102, 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zampighi, G. A., Loo, D. D., Kreman, M., Eskandari, S. & Wright, E. M. (1999) J. Gen. Physiol. 113, 507–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trexler, E. B., Bukauskas, F. F., Bennett, M. V. L., Bargiello, T. A. & Verselis, V. K. (1999) J. Gen. Physiol. 113, 721–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eskandari, S., Zampighi, G. A., Leung, D. W., Wright, E. M. & Loo, D. D. (2002) J. Membr. Biol. 185, 93–102. [DOI] [PubMed] [Google Scholar]
- 26.Trexler, E. B., Bennett, M. V. L., Bargiello, T. A. & Verselis, V. K. (1996) Proc. Natl. Acad. Sci. USA 93, 5836–5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trexler, E. B., Bukauskas, F. F., Kronengold, J., Bargiello, T. A. & Verselis, V. K. (2000) Biophys. J. 79, 3036–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bukauskas, F. F., Bukauskiene, A., Bennett, M. V. L. & Verselis, V. K. (2001) Biophys. J. 81, 137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan, K., Solan, J. L., Dominguez, M., Sia, M., Hand, A., Lampe, P. D. & Laird, D. W. (1999) Mol. Biol. Cell 10, 2033–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bukauskas, F. F., Jordan, K., Bukauskiene, A., Bennett, M. V. L., Lampe, P. D., Laird, D. W. & Verselis, V. K. (2000) Proc. Natl. Acad. Sci. USA 97, 2556–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bukauskas, F. F. (2001) Methods Mol. Biol. 154, 379–393. [DOI] [PubMed] [Google Scholar]
- 32.Naus, C. C., Bond, S. L., Bechberger, J. F. & Rushlow, W. (2000) Brain Res. Brain Res. Rev. 32, 259–266. [DOI] [PubMed] [Google Scholar]
- 33.Schubert, A. L., Schubert, W., Spray, D. C. & Lisanti, M. P. (2002) Biochemistry 41, 5754–5764. [DOI] [PubMed] [Google Scholar]
- 34.Giepmans, B. N. & Moolenaar, W. H. (1998) Curr. Biol. 8, 931–934. [DOI] [PubMed] [Google Scholar]
- 35.Bennett, M. V. L., Contreras, J. E., Bukauskas, F. F. & Sáez, J. C. (2003) Trends Neurosci., in press. [DOI] [PMC free article] [PubMed]
- 36.Verselis, V. K. & Bukauskas, F. F. (2002) Curr. Drug Targets 3, 83–99. [DOI] [PubMed] [Google Scholar]
- 37.Martin, P. E., George, C. H., Castro, C., Kendall, J. M., Capel, J., Campbell, A. K., Revilla, A., Barrio, L. C. & Evans, W. H. (1998) J. Biol. Chem. 273, 1719–1726. [DOI] [PubMed] [Google Scholar]
- 38.Revilla, A., Castro, C. & Barrio, L. C. (1999) Biophys. J. 77, 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh, S., Rubin, J. B., Bennett, M. V. L., Verselis, V. K. & Bargiello, T. A. (1999) J. Gen. Physiol. 114, 339–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purnick, P. E., Oh, S., Abrams, C. K., Verselis, V. K. & Bargiello, T. A. (2000) Biophys. J. 79, 2403–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh, S., Abrams, C. K., Verselis, V. K. & Bargiello, T. A. (2000) J. Gen. Physiol. 116, 13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romanello, M. & D'Andrea, P. (2001) J. Bone Miner. Res. 16, 1465–1476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.