Abstract
It is becoming increasingly clear that signaling via G protein-coupled receptors is a diverse phenomenon involving receptor interaction with a variety of signaling partners. Despite this diversity, receptor ligands are commonly classified only according to their ability to modify G protein-dependent signaling. Here we show that β2AR ligands like ICI118551 and propranolol, which are inverse agonists for Gs-stimulated adenylyl cyclase, induce partial agonist responses for the mitogen-activated protein kinases extracellular signal-regulated kinase (ERK) 1/2 thus behaving as dual efficacy ligands. ERK1/2 activation by dual efficacy ligands was not affected by ADP-ribosylation of Gαi and could be observed in S49-cyc– cells lacking Gαs indicating that, unlike the conventional agonist isoproterenol, these drugs induce ERK1/2 activation in a Gs/i-independent manner. In contrast, this activation was inhibited by a dominant negative mutant of β-arrestin and was abolished in mouse embryonic fibroblasts lacking β-arrestin 1 and 2. The role of β-arrestin was further confirmed by showing that transfection of β-arrestin 2 in these knockout cells restored ICI118551 promoted ERK1/2 activation. ICI118551 and propranolol also promoted β-arrestin recruitment to the receptor. Taken together, these observations suggest that β-arrestin recruitment is not an exclusive property of agonists, and that ligands classically classified as inverse agonists rely exclusively on β-arrestin for their positive signaling activity. This phenomenon is not unique to β2-adrenergic ligands because SR121463B, an inverse agonist on the V2 vasopressin receptor-stimulated adenylyl cyclase, recruited β-arrestin and stimulated ERK1/2. These results point to a multistate model of receptor activation in which ligand-specific conformations are capable of differentially activating distinct signaling partners.
The signaling unit for G protein-coupled receptors (GPCRs) has been classically defined as consisting of the receptor, an heterotrimeric G protein and enzymatic or channel effectors controlling second messenger production or ion fluxes that regulate cell function. More recently it has become clear that GPCRs may also stimulate a variety of signaling systems that regulate cell proliferation and differentiation such as mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinase (ERK) 1/2, c-Jun-NH2-terminal kinase (JNK) 3, and p38 (1). In many cases, mitogenic signals generated by GPCRs involve classical second messenger-dependent pathways (2), but in others, they require the formation of metastable complexes that comprise the receptor, accessory proteins such as β-arrestins or ASK1 and effectors molecules such as Src, Ras, ERK1/2, JNK, and MAPK kinase 4 (MKK4) (3–6).
Despite this growing diversity in GPCR signaling mechanisms, our definition of drug efficacy is still linked to a scheme considering only the regulation of the classical G protein signaling. Within this framework, agonists are defined as drugs that stabilize an active receptor conformation that induces G protein activation, whereas inverse agonists favor an inactive receptor state that reduces spontaneous G protein signaling (7). The question that now arises is whether this same paradigm may be transferred to drug effects generated through the formation of metastable complexes involving scaffolding proteins such as β-arrestin. Because all studies describing β-arrestin-mediated MAPK signaling have concentrated on agonist drugs, little is known of how ligands that are commonly classified as inverse agonists may regulate the scaffold assembly that is crucial for such signaling.
In the present study, this question was addressed by assessing whether β2-adrenergic receptor (β2AR) ligands with proven inverse efficacy on adenylyl cyclase (AC) activity could also regulate MAPK activation via receptor-mediated scaffold formation. We found that, despite being inverse agonists in the AC pathway, ICI118551, and propranolol induced the recruitment of β-arrestin leading to the activation of the ERK cascade. Such observations indicate that the same drug acting on a unique receptor can have opposite efficacies depending on the signaling pathway considered.
Materials and Methods
Expression Vectors. Wild-type β2AR and V2 vasopressin receptor (V2R). Wild-type human β2AR, V2R, and hemagglutinin (HA)-tagged (YPYDVPDYA) β2AR sequences were subcloned into a modified pcDNA3 vector (Invitrogen) in which the cytomegalovirus promoter was replaced by a Rous sarcoma virus promoter as described (8).
V2R-GFP and β2AR-GFP. The coding sequence of the green fluorescent protein variant GFP 10 (9) was inserted in-frame 3′ of the wild-type V2R sequence into pcDNA3-V2R so that a 14-aa linker (GSGTAGPGSPPVAT), separates the carboxyl tail of the receptor and the initiator methionine of GFP10. For the β2AR-GFP, a GFP10 AgeI/BsrgI fragment was subcloned into the AgeI/BsrgI site of pGFP-N1-Hisβ2AR-YFP (10) to yield pGFP-N1–Hisβ2AR-GFP10.
β-Arrestin-Rluc. The rat β-arrestin 2 coding sequence was inserted in-frame 5′of the Renilla luciferase sequence in the pRluc vector (Perkin-Elmer) so that a 7-aa linker (GAGALAT) separates the carboxyl terminal of β-arrestin and the initiator methionine of Rluc.
β-Arrestin-YFP. The rat β-arrestin 2 coding sequence was amplified out of its original vector with two NheI site-containing primers to generate a stop codon-free fragment that was then subcloned in-frame 3′ into the NheI site of a yellow variant GFP-topaz vector (pGFP-N1-Topaz, Packard; ref. 10). All constructs were confirmed by sequencing.
Cell Culture and Transfections. Mouse embryonic fibroblasts (MEFs), HEK293 and Cos1 cells were grown in DMEM supplemented with 10% FBS, 2 mM glutamine, 0.1 units/ml penicillin, 0.1 mg/ml streptomycin, and 0.25 μg/ml fungizone. S49 mouse lymphoma T cells were grown in DMEM as above but supplemented with 10% heat-inactivated horse serum. Freshly dispersed cardiomyocytes were prepared as described (11). Calcium phosphate precipitation and G418 selection pressure (450 μg/ml) were used to produce stable HEK293 cell lines expressing β2AR or V2R. MEFs were transiently transfected by using FuGENE 6 (Roche Molecular Biochemicals) according to supplier's specifications. All other transient transfections were carried out by calcium phosphate precipitation. Cells were cultivated under sterile conditions at 37°C in a 5% CO2 atmosphere.
MAPK Activity, cAMP Accumulation, and Gene Reporter Assays. For ERK1/2 phosphorylation assays, cells were grown in six-well plates, serum-starved overnight and then incubated in PBS, at 37°C in the presence of different ligands. Cells were lysed in sample buffer and separated on SDS/10% PAGE, according to the method of Laemmli (12). Proteins were transferred onto nitrocellulose and Western blot analysis carried out by using a 1:5,000 dilution of mouse monoclonal antiphospho-ERK1/2 antibody (Santa Cruz Biotechnology). Total ERK protein was determined by using a 1:20,000 dilution of anti-ERK antibody (Santa Cruz Biotechnology). Blots were then probed with anti-mouse or anti-rabbit horseradish peroxydase-conjugated secondary antibodies, visualized by using enhanced chemiluminescence reagent (Amersham Pharmacia) and quantified by densitometric analysis. ERK1/2 phosphorylation was normalized according to the loading of proteins by expressing the data as a ratio of phospho-ERK1/2 over total ERK1/2. Results were expressed as percentage of the value obtained in nonstimulated cells.
For cAMP accumulation assays, cells were incubated for 16 h with [3H]adenine (2 μCi/ml; 1 Ci = 37 GBq) and washed twice before stimulation with the indicated drugs at 37°C. After a 15-min incubation, the reaction was stopped by adding 1 ml of ice-cold 5% trichloroacetic acid and 1 mM of unlabeled cAMP. [3H]cAMP was then separated from [3H]ATP by sequential chromatography over Dowex and Alumina columns. cAMP accumulation was calculated as ([3H]cAMP cpm/([3H]cAMP cpm + [3H]ATP cpm)) × 1,000 and expressed as a percentage of nonstimulated cells.
For gene reporter assays, HEK293 cells stably expressing the β2AR were transiently transfected with pFA-Elk1 and pFR-Luc reporter plasmids of the PathDetect Reporting System (Stratagene), by using calcium phosphate precipitation procedure. After a 6-h incubation with the indicated ligands, luciferase activity was measured in cell lysates by using a firefly luciferase assay kit (Promega) and a LumiCount luminometer (Packard), as described (13).
Bioluminescence Resonance Energy Transfer (BRET) Assay. BRET is a proximity assay based on the nonradiative transfer of energy between a donor (luciferase) and an acceptor (GFP). It was used to assess β-arrestin recruitment to β2AR or V2R as described (10). Briefly, fusion proteins consisting of the GFP-10 variant (9), covalently attached to the carboxyl tail of the receptor of interest (β2AR-GFP; V2R-GFP), were coexpressed with β-arrestin 2 fused at its carboxyl terminus to Rluc (β-arrestin-Rluc). After incubation of the transfected cells with different ligands, Deep Blue coelanterazine (Packard) was added at a final concentration of 5 μM and readings were collected by using a modified top-count apparatus (BRETCount, Packard) that allows the sequential integration of the signals detected in the 370- to 450- and 500- to 530-nm windows. The BRET signal was determined by calculating the ratio of the light emitted by the Receptor-GFP (500–530 nm) over the light emitted by the β-arrestin-2-Rluc (370–450 nm). The values were corrected by subtracting the background signal detected when the β-arrestin-2-Rluc construct was expressed alone.
Immunofluorescence and Confocal Microscopy. Immunofluorescence microscopy was carried out in Cos1 cells transiently transfected either with a HA-tagged β2V2R chimera (consisting of the first 341 aa of the β2AR and the last 29 aa of the V2) and β-arrestin-2–GFP or HA-tagged β2AR and ERK2 GFP by using FuGENE 6. Immunolabeling of the HA-tagged β2V2R and β2AR was carried out in nonpermeabilized cells by using the anti-HA (12CA5; 1:100) antibody. Immunoreactivity was revealed by using a Texas red-conjugated secondary antibody. β-arrestin-GFP and ERK2-GFP were visualized by direct fluorescence detection. Quantitative assessment of nuclear translocation of ERK2-GFP was carried out by counting the number of cells in which GFP fluorescence colocalized with the nuclear marker To-Pro-3 iodine (Molecular Probes). All confocal microscopy images were acquired on a Leica HM IRBE laser-scanning microscope with a ×100 objective.
Results and Discussion
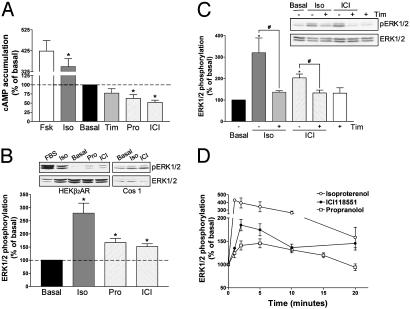
HEK293 fibroblasts stably expressing the β2AR were used to compare the effects of different β2AR ligands on the classical Gαs-mediated activation of the AC pathway and the MAPK cascade. As previously reported (14, 15), isoproterenol behaved as an agonist in the two systems, leading to increased cAMP production and ERK1/2 phosphorylation (Fig. 1 A and B). Also in agreement with previous reports (14), propranolol and ICI118551 produced inverse agonist effects for the AC pathway, inhibiting cAMP production by 35% and 50%, respectively (Fig. 1 A). However, these two compounds were found to be agonists at the MAPK cascade, with propranolol inducing a 67% increase and ICI118551 inducing a 52% increase in ERK1/2 phosphorylation (Fig. 1B). Timolol, was devoid of intrinsic activity in the two pathways (Fig. 1 A and C) but competitively blocked both inverse agonist (ref. 14 and data not shown) and agonist (Fig. 1C) effects of ICI118551.
Fig. 1.
Signaling efficacy of β2AR ligands. HEK293 cells stably expressing the β2AR (HEKβ2AR) were treated with the indicated drugs (1 μM), and cAMP accumulation (A) and ERK1/2 phosphorylation (B) were assessed after a 15-min and a 5-min incubation, respectively. Results are expressed as percentage of the value obtained in nonstimulated cells and represent the mean ± SEM of three to eight independent experiments. *, P < 0.01 compared with basal. (B Inset) Representative examples of ERK1/2 phosphorylation experiments carried out in HEKβ2AR and Cos1 cells. (C) Competitive antagonism of timolol (10 μM) on isoproterenol-mediated (10 nM) and ICI118551-mediated (10 nM) ERK1/2 phosphorylation (n = 8–10; *, P < 0.05 compared with basal cells; #, P < 0.05 for the indicated comparison). (D) Kinetics of ERK1/2 activation by different β2AR ligands (1 μM) in HEKβ2AR (circles) and canine cardiomyocytes (squares) (n = 4–7).
Opposing effects of ICI118551 and propranolol on AC and MAPK activity cannot be attributed to an artifact related to receptor overexpression in HEK293 cells because their dual actions were also observed in Cos1 (Fig. 1B Inset), S49 cells (see below), and cardiomyocytes (Fig. 1D) all of them endogenously expressing low levels of β2AR. The activation kinetics of MAPK by isoproterenol, ICI118551, and propranolol were similar; activation was fast and transient, and reached a peak between 1 and 2 min after drug addition (Fig. 1D). However, the effects of ICI118551 and propranolol appear to be slightly slower because their maximal activity was observed at 2 min rather than at 1 min, as for isoproterenol.
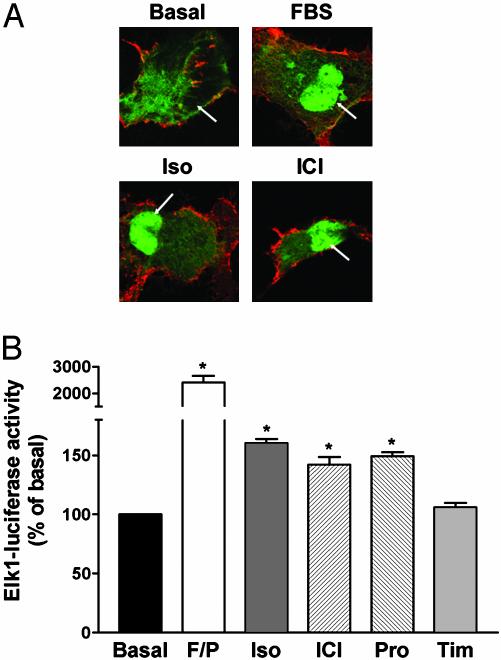
Consistent with the known longer-term nuclear translocation of activated ERK, both isoproterenol and ICI118551 promoted targeting of ERK2-GFP to the nucleus after a 20-min treatment with the drugs (Fig. 2A). As is the case for isoproterenol, ERK nuclear translocation promoted by dual efficacy drugs ICI118551 and propranolol also has functional consequences as illustrated by increased Elk-dependent transcriptional activity (Fig. 2B). Given the role played by ERK in β-adrenergic-mediated cardiac fibroblast hypertrophy and the general use of β2AR antagonists and inverse agonists in the treatment of hypertension and heart failure (16), the agonistic properties of these compounds on the ERK pathway could have clinically relevant implications.
Fig. 2.
Nuclear translocation of activated ERK. (A) Subcellular redistribution of ERK2-GFP after FBS, isoproterenol (Iso) and ICI118551 (ICI) treatments. Cos1 cells transiently expressing HA-β2AR and ERK2-GFP were stimulated or not for 20 min with 10% FBS or 10 μM Iso or ICI. Arrows indicate the nucleus. Under basal conditions, 13% of the cells showed colocalization of ERK2-GFP with the nuclear marker To-Pro-3 iodine. After drug treatments, this value increased to 84% for FBS, 72% for Iso, and 67% for ICI. (B) Gene reporter assay for Elk-regulated transcription. Luciferase activity was measured after a 6-h incubation with 10 μM Iso, ICI, propranolol (Pro), or timolol (Tim). Stimulation with 10% FBS and 1 μM phorbol 12-myristate 13-acetate (F/P) was used as a positive control. Data are expressed as percentage of the value obtained in nonstimulated cells and represent the mean ± SEM of four to six independent experiments; *, P < 0.01 compared with basal.
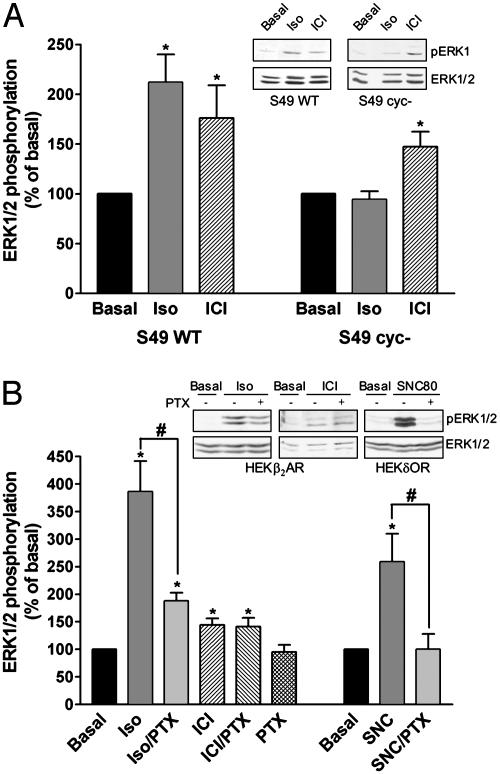
Because both Gαs (2, 15, 17) and Gαi (15, 18) have been shown to contribute to MAPK activation by the conventional agonist isoproterenol, we sought to determine whether this was also the case for drugs with dual efficacy like propranolol or ICI118551. The possible role of Gαs was assessed by comparing the effects of ICI118551 and isoproterenol in S49 wild-type and S49 cyc– cells that lack Gαs (19). As shown in Fig. 3A, both isoproterenol and ICI118551 promoted ERK phosphorylation in wild-type S49 cells, but only ICI118551 activated the kinase in the cyc– cells, indicating that Gαs is not needed for MAPK activation by dual efficacy drugs. To test the potential implication of Gαi/o, HEK293 cells were pretreated with pertussis toxin (PTX). The treatment abolished MAPK activation by a typical Gαi/o coupled δ-opioid receptor but was without effect on ERK phosphorylation induced by ICI118551 (Fig. 3B), ruling out a role for Gαi/o in this response. In keeping with previous observations (15, 18) where MAPK activation by isoproterenol has been shown to require the sequential activation of Gαs and Gαi/o, the agonist response was not only absent in the S49 cyc– cells, but was also significantly inhibited by PTX treatment.
Fig. 3.
Role of Gs and Gi in isoproterenol and ICI118551-mediated ERK1/2 activation. ERK1/2 phosphorylation was assessed in S49 wild-type (S49 WT) and S49 cyc–cells endogenously expressing the β2AR (A) or in HEK293 cells stably transfected with the β2AR (HEKβ2AR) and treated or not with 100 ng/ml pertussis toxin (PTX) for 16 h (B). Cells were stimulated with 1 μM isoproterenol (Iso) or ICI118551 (ICI) for 5 min. The efficacy of PTX pretreatment was controlled by its ability to block ERK1/2 activation in HEK293 cells expressing the Gi/o-coupled δ-opioid receptor (HEKδOR) by the δOR agonist SNC80 (1 μM; 5 min). Results are expressed as percentage of the value obtained in nonstimulated cells and represent the mean ± SEM of three to eight independent experiments. *, P < 0.05 compared with basal; #, P < 0.05 for the indicated comparison. Insets show representative experiments.
Taken together, these results indicate that unlike isoproterenol, ICI118551 activates the MAPK signaling pathway independently of Gαi and Gαs. The lack of G protein involvement is further supported by the observation that overexpression of the carboxyl tail of GRK2, which acts as a scavenger of Gβγ (20), did not modify ICI118551-stimulated ERK1/2 phosphorylation (data not shown). G protein-independent activation of MAPK has been recently documented for mutants of the angiotensin II type 1 receptors that fail to couple to their cognate G proteins (21). This finding may indicate that G protein-independent MAPK signaling is a general feature of heptahelical receptors, unveiled when G protein coupling is prevented either as a result of mutations or after the stabilization of the receptor by ligands such as ICI118551 or propranolol that inhibit receptor–G protein interaction.
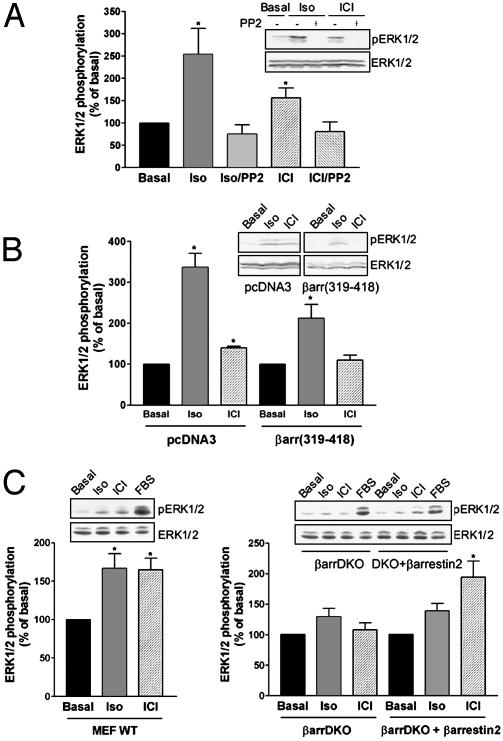
The nonreceptor tyrosine kinase Src is an intermediate in diverse pathways leading to MAPK activation by GPCRs (1, 3, 22) whether they involve G protein signaling (15) or not (21). As shown in Fig. 4A, G protein-independent activation of ERK by ICI118551 was also found to involve Src activation because it was blocked by the selective Src inhibitor PP2. Several mechanisms of Src activation by GPCRs have been proposed (1). For the β2AR, isoproterenol stimulation has been shown to promote the recruitment of Src through an indirect interaction that involves the proline-rich motif of β-arrestin and the Src homology 3 domain of Src (3). In addition to its adaptor role, bridging the receptor and Src, β-arrestin has been shown to function as a scaffold protein organizing the assembly of Raf, MAP/ERK kinase, ERK/ASK1, MKK4, and JNK3 modules for an increasing number of GPCRs (4–6, 23). We therefore examined the role of β-arrestin in promoting ERK1/2 activity by ICI118551. To do so, we first used a dominant negative mutant corresponding to the carboxyl-terminal fragment (βarr319–418) of β-arrestin, previously shown to decrease ERK1/2 activation by isoproterenol (24). Similar to the isoproterenol response, ICI118551 activation of ERK1/2 was inhibited by βarr319–418 (Fig. 4B), suggesting that β-arrestin also mediates the MAPK stimulation by dual efficacy drugs. The role of β-arrestin in ERK1/2-activation by ICI118551 was further documented by using MEFs derived from knockout (KO) animals lacking β-arrestin 1 and 2 (βarr-DKO) (25). In contrast with the significant ERK phosphorylation induced by isoproterenol and ICI118551 in wild-type MEFs, neither drug activated the MAPK pathway in the βarr-DKO cells (Fig. 4C). However, the ERK1/2 response to ICI118551 was restored on transient transfection of β-arrestin 2 into double KO (DKO) cells (Fig. 4C) confirming its role as an adaptor/scaffolding protein in this pathway.
Fig. 4.
Involvement of Src and β-arrestin in isoproterenol- and ICI118551-mediated ERK1/2 activation. ERK1/2 phosphorylation was assessed in HEK293 cells stably expressing the β2AR pretreated or not with the cell-permeable Src tyrosine kinase inhibitor, PP2 (50 μM, 1 h) (A), or transiently transfected with the β-arrestin dominant negative βarr(319–418) or the empty vector pcDNA3 as a control (B). (C) ERK1/2 phosphorylation was assessed in mouse embryonic fibroblasts (MEF) derived from wild-type mice (WT) and from KO mice lacking both β-arrestin 1 and 2 (βarrDKO) that were transiently transfected with the human HA-β2AR. Expression levels of 150–250 fmol of β2AR per mg of protein were reached in both WT and KO cells. For rescue experiments, βarrDKO MEF were cotransfected with HA-β2AR and β-arrestin 2 (βarrDKO + β-arrestin 2). In all cases, cells were stimulated for 5 min with 1 μM isoproterenol (Iso) or ICI118551 (ICI). Data are expressed as percentage of the value obtained in nonstimulated cells and represent the mean ± SEM of three to eight independent experiments. *, P < 0.05 compared with basal. Insets show representative experiments.
β-arrestins have been extensively characterized as regulators of agonist-induced GPCR signaling (26), and their role in agonist-promoted desensitization and receptor internalization are well defined. Classically, β-arrestins are recruited to the membrane by the agonist-occupied state of the receptor. Once this association takes place, the receptor is uncoupled from the G protein (27) and targeted to the endocytic compartment (28) via direct interactions with proteins of clathrin-coated pits (29–31). The observation that ICI118551 relies on β-arrestin to induce MAPK activation suggests that, in addition to classical agonists, some drugs normally classified as “inverse agonists” can also promote the recruitment of β-arrestin.
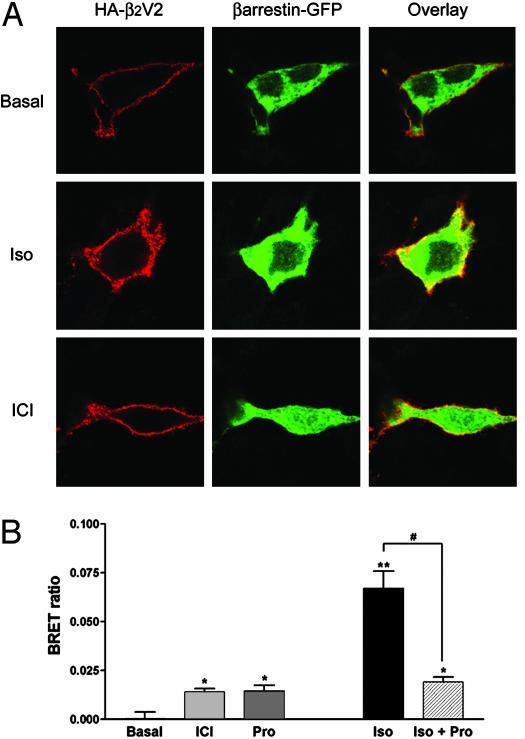
This hypothesis was tested by assessing ligand-promoted β-arrestin recruitment using immunofluorescence microscopy. In Cos1 cells coexpressing HA-tagged β2AR and β-arrestin-GFP, a modest translocation of β-arrestin to the membrane could be observed on stimulation with isoproterenol but not ICI118551 or propranolol (data not shown). We reasoned that this negative result could be caused by a combination of factors. First, the β2AR belongs to a class of GPCRs known to have relatively low affinity for β-arrestin, leading to only transient complexes between the two proteins (31). Second, when compared with isoproterenol, ICI118551 and propranolol display partial agonistic activities toward MAPK signaling and their reduced efficacy could have caused incomplete β-arrestin recruitment that went undetected. To overcome these limitations, we took advantage of the fact that V2R interacts more tightly than β2AR with β-arrestin (31). Because this property has been mapped to the last 10 aa of the V2R tail, we used a receptor chimera consisting of the first 341 aa of the β2AR fused to the last 29 aa of the V2R. This chimera has previously been shown to have high affinity for β-arrestin (31). As shown in Fig. 5A, isoproterenol and ICI118551 promoted a robust translocation of β-arrestin-GFP to the membrane of Cos1 cells expressing the chimeric β2V2R. In contrast, vasopressin, which does not bind the chimera, was without effect on β-arrestin cell distribution (data not shown).
Fig. 5.
β-arrestin recruitment. (A) Cos1 cells transiently expressing the HA-tagged β2V2R chimera and β-arrestin-2-GFP were stimulated or not for 15 min with 10 μM isoproterenol (Iso) or ICI118551 (ICI). Subcellular distribution was then assessed by fluorescence microscopy using Texas red-conjugated antibodies for the HA-β2V2R and direct fluorescence for the β-arrestin-GFP. (B) BRET was determined in HEK293 cells transiently coexpressing β2AR-GFP and β-arrestin-Rluc after incubation with 1 μM isoproterenol (Iso), ICI118551 (ICI), or propranolol (Pro) for 1 h at room temperature. Competitive antagonism of Pro on Iso-mediated β-arrestin recruitment was measured after pretreatment of the cells with 10 μM Pro for 15 min before stimulation with 0.1 μM Iso. Data are expressed as BRET ratio and represent the mean ± SEM of four independent experiments. *, P < 0.05 compared with nonstimulated cells (basal); **, P < 0.01 compared with nonstimulated cells (basal); #, P < 0.001 for the indicated comparison.
The results presented above are consistent with the notion that binding of compounds such as ICI118551 and propranolol can stabilize a receptor conformation that allows β-arrestin binding to a chimeric β2V2R receptor, but did not prove that this can occur for the wild-type β2AR. To directly assess the physical interaction between the ligand-bound β2AR itself and β-arrestin, we turned to a more sensitive assay based on BRET. For this, β2AR-GFP and β-arrestin-Rluc were transiently coexpressed in HEK293 cells. As shown in Fig. 5B, isoproterenol, propranolol, and ICI118551 all induced β-arrestin recruitment, as indicated by an increase in the BRET signal. Consistent with the partial agonist effect of ICI118551 and propranolol on the MAPK activity, BRET increases promoted by these compounds were significantly smaller than the one induced by isoproterenol. This low efficacy of propranolol in promoting β-arrestin recruitment is consistent with previous immunofluorescence results showing that propranolol can inhibit isoproterenol-induced β-arrestin mobilization (29). Such an inhibition is also seen in the BRET experiments where propranolol reduced the signal induced by isoproterenol to the level attained on stimulation with propranolol alone (Fig. 5B).
Taken together, our observations suggest that, although they are inverse agonists on the Gαs-stimulated AC cascade, compounds such as ICI118551 and propranolol have partial agonist efficacy on the β-arrestin-dependent MAPK pathway. These findings are not consistent with a classical view proposing a unique “activated” receptor conformation involved in G protein coupling and β-arrestin recruitment. They are more in keeping with the emerging concept that distinct active conformations may be stabilized by different ligands (32) and that some of these conformations may favor activation of one effector over another (33) or induce differential recruitment of β-arrestin (34, 35). For the systems considered herein, classical agonists like isoproterenol would stabilize a receptor state capable of activating Gαs and of promoting the recruitment of β-arrestin, whereas the conformation stabilized by ICI118551 or propranolol would interdict productive G protein coupling but favor β-arrestin engagement. Previous reports showing that some GPCR antagonists can promote receptor endocytosis (36, 37), a phenomenon normally triggered by agonists, could also find their explanation in the concept of discrete receptor conformations that have only some of the properties of the fully active state. The idea that β-arrestin may interact with several distinct receptor conformations is also consistent with the observations that not only agonists (38) but also certain GPCR antagonists (39) display high affinity for the β-arrestin-bound form of the receptor.
In addition to demonstrating that ligands may have dual efficacies depending on the effector system considered, our study clearly shows that a single receptor can activate the MAPK through distinct mechanisms. Indeed, although both Gs and β-arrestin were found to be involved in the isoproterenol-mediated activation, only β-arrestin contributes to the activation by ICI118551 and propranolol. Whether this difference in the MAPK activation mechanism between the two classes of ligands could underlie the modest difference in the activation kinetics observed in Fig. 1D remains to be determined.
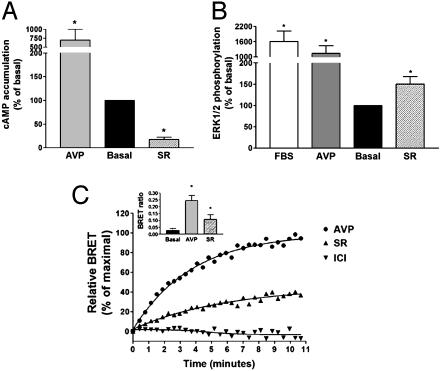
To assess whether dual efficacy was unique to β2AR ligands or whether it could be a general concept applicable to other seven transmembrane domain receptors, AC and MAPK regulation by V2R drugs was also examined. As shown in Fig. 6 A and B, SR121463B, a non-peptide V2R selective ligand, induced an inverse agonist response on the AC pathway but clearly stimulated MAPK activity in HEK293 cells stably expressing the human V2R, an observation that extends the concept of dual efficacy ligands to another GPCR. Similarly to what was observed for ICI118551 and propranolol at the β2AR, SR121463B promoted the recruitment of β-arrestin to the V2R. Indeed, a time-dependent recruitment could be detected by BRET in HEK293 cells transiently coexpressing V2R-GFP and β-arrestin-Rluc (Fig. 6C). In agreement with the partial agonist activity of SR121463B on the MAPK activity, its β-arrestin recruitment was also partial, with a BRET signal that reached 38% of the vasopressin-induced response. As expected, ICI118551 was without effect on the recruitment of β-arrestin to the V2R, confirming the selectivity of the response.
Fig. 6.
MAPK activation and β-arrestin recruitment by the V2R ligand SR121463B. HEK293 cells stably expressing the V2R were treated with 1 μM Arg-vasopressin (AVP), SR121463B (SR), or 10% FBS, and cAMP accumulation (A) and ERK1/2 phosphorylation (B) were assessed after 15 and 5 min of incubation, respectively. Results are expressed as percentage of the value obtained in nonstimulated cells and represent the mean ± SEM of three to four independent experiments. *, P < 0.05 compared with basal. (C) BRET was determined in HEK293 cells transiently coexpressing V2R-GFP and β-arrestin-Rluc in the presence of 1 μM AVP (•), SR (▴), or ICI (▾) for the indicated times. Data are expressed as percentage of the maximal BRET signal promoted by AVP and are representative of two to four independent experiments. (Inset) BRET ratio after a 5-min stimulation with 1 μM AVP or SR. Data represent the mean ± SEM of four independent experiments. *, P < 0.05 compared with nonstimulated cells (basal).
Overall, the results of the present study point to a general multistate model of receptor activation in which ligand-specific conformations are capable of differential activation of distinct signaling partners. This is reminiscent of the concept of “trafficking of receptor stimulus” (33, 40) previously proposed to explain that ligands can activate diverse effectors with distinct rank order of potencies and/or efficacies. However, results presented herein add a new level of complexity, showing that a ligand can have opposing efficacies on two effector systems engaged by the same receptor, indicating that the “direction” of response can be influenced by the effector considered. The study also confirms β-arrestin in its role as a genuine signaling partner that can be involved in the G protein-independent signaling of seven transmembrane domain receptors.
Acknowledgments
We thank M. Lagacé for critical reading of the manuscript and A. Laperriere for expert technical assistance. MEF β-arrestin KO cells were a gift of R. J. Lefkowitz [Howard Hughes Medical Institute (HHMI), Duke University, Durham, NC], the β2V2R construct was kindly provided by M. Caron (HHMI, Duke University), ERK2-GFP by K. DeFea (University of California, San Francisco), and rat β-arrestin 2 by S. Ferguson (Roberts Research Institute, University of Western Ontario, London, ON, Canada). This work was supported by grants from the Canadian Institute for Health Research and the Heart and Stroke Foundation of Canada. M.A. retains a fellowship from the Canadian Hypertension Society, and P.G.C. retains a studentship from the Heart and Stroke Foundation of Canada and Fonds de Recherche en Sante du Quebec (FRSQ); G.P. is recipient of a fellowship from FRSQ; and M.B. holds a Canada Chair in Signal Transduction and Molecular Pharmacology.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GPCRs, G protein-coupled receptors; MAPK, mitogen-activated protein kinase; β2AR, β2-adrenergic receptor; AC, adenylyl cyclase; ERK, extracellular-regulated kinase; MEF, mouse embryonic fibroblast; V2R, type 2 vasopressin receptor; HA, hemagglutinin; BRET, bioluminescence resonance energy transfer. KO, knockout; DKO, double KO.
References
- 1.Gutkind, J. S. (2000) Sci. STKE 40, RE1. [DOI] [PubMed] [Google Scholar]
- 2.Schmitt, J. M. & Stork, P. J. (2000) J. Biol. Chem. 275, 25342–25350. [DOI] [PubMed] [Google Scholar]
- 3.Luttrell, L. M., Ferguson, S. S., Daaka, Y., Miller, W. E., Maudsley, S., Della Rocca, G. J., Lin, F., Kawakatsu, H., Owada, K., Luttrell, D. K., et al. (1999) Science 283, 655–661. [DOI] [PubMed] [Google Scholar]
- 4.DeFea, K. A., Zalevsky, J., Thoma, M. S., Dery, O., Mullins, R. D. & Bunnett, N. W. (2000) J. Cell. Biol. 148, 1267–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald, P. H., Chow, C. W., Miller, W. E., Laporte, S. A., Field, M. E., Lin, F. T., Davis, R. J. & Lefkowitz, R. J. (2000) Science 290, 1574–1577. [DOI] [PubMed] [Google Scholar]
- 6.Luttrell, L. M., Roudabush, F. L., Choy, E. W., Miller, W. E., Field, M. E., Pierce, K. L. & Lefkowitz, R. J. (2001) Proc. Natl. Acad. Sci. USA 98, 2449–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenakin, T. (2001) FASEB J. 15, 598–611. [DOI] [PubMed] [Google Scholar]
- 8.Adam, L., Bouvier, M. & Jones, T. L. (1999) J. Biol. Chem. 274, 26337–26343. [DOI] [PubMed] [Google Scholar]
- 9.Mercier, J. F., Salahpour, A., Angers, S., Breit, A. & Bouvier, M. (2002) J. Biol. Chem. 277, 44925–44931. [DOI] [PubMed] [Google Scholar]
- 10.Angers, S., Salahpour, A., Joly, E., Hilairet, S., Chelsky, D., Dennis, M. & Bouvier, M. (2000) Proc. Natl. Acad. Sci. USA 97, 3684–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurent, C. E., Cardinal, R., Rousseau, G., Vermeulen, M., Bouchard, C., Wilkinson, M., Armour, J. A. & Bouvier, M. (2001) Am. J. Physiol. 80, R355–R364. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli, U. K. (1970) Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- 13.Blaukat, A., Pizard, A., Rajerison, R. M., Alhenc-Gelas, F., Muller-Esterl, W. & Dikic, I. (1999) FEBS Lett. 451, 337–341. [DOI] [PubMed] [Google Scholar]
- 14.Chidiac, P., Hebert, T. E., Valiquette, M., Dennis, M. & Bouvier, M. (1994) Mol. Pharmacol. 45, 490–499. [PubMed] [Google Scholar]
- 15.Daaka, Y., Luttrell, L. M. & Lefkowitz, R. J. (1997) Nature 390, 88–91. [DOI] [PubMed] [Google Scholar]
- 16.Murray, D. R. & Dugan, J. (2000) Cardiol. Rev. 8, 340–347. [DOI] [PubMed] [Google Scholar]
- 17.Wan, Y. & Huang, X. Y. (1998) J. Biol. Chem. 273, 14533–14537. [DOI] [PubMed] [Google Scholar]
- 18.Zamah, A. M., Delahunty, M., Luttrell, L. M. & Lefkowitz, R. J. (2002) J. Biol. Chem. 277, 31249–31256. [DOI] [PubMed] [Google Scholar]
- 19.Salomon, M. R. & Bourne, H. R. (1981) Mol. Pharmacol. 19, 109–116. [PubMed] [Google Scholar]
- 20.Luttrell, L. M., van Biesen, T., Hawes, B. E., Koch, W. J., Touhara, K. & Lefkowitz, R. J. (1995) J. Biol. Chem. 270, 16495–16498. [DOI] [PubMed] [Google Scholar]
- 21.Seta, K., Nanamori, M., Modrall, J. G., Neubig, R. R. & Sadoshima, J. (2002) J. Biol. Chem. 277, 9268–9277. [DOI] [PubMed] [Google Scholar]
- 22.Maudsley, S., Pierce, K. L., Zamah, A. M., Miller, W. E., Ahn, S., Daaka, Y., Lefkowitz, R. J. & Luttrell, L. M. (2000) J. Biol. Chem. 275, 9572–9580. [DOI] [PubMed] [Google Scholar]
- 23.DeFea, K. A., Vaughn, Z. D., O'Bryan, E. M., Nishijima, D., Dery, O. & Bunnett, N. W. (2000) Proc. Natl. Acad. Sci. USA 97, 11086–11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce, K. L., Maudsley, S., Daaka, Y., Luttrell, L. M. & Lefkowitz, R. J. (2000) Proc. Natl. Acad. Sci. USA 97, 1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohout, T. A., Lin, F. S., Perry, S. J., Conner, D. A. & Lefkowitz, R. J. (2001) Proc. Natl. Acad. Sci. USA 98, 1601–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierce, K. L. & Lefkowitz, R. J. (2001) Nat. Rev. Neurosci. 2, 727–733. [DOI] [PubMed] [Google Scholar]
- 27.Benovic, J. L., Bouvier, M., Caron, M. G. & Lefkowitz, R. J. (1988) Annu. Rev. Cell. Biol. 4, 405–428. [DOI] [PubMed] [Google Scholar]
- 28.Scott, M. G., Benmerah, A., Muntaner O. & Marullo, S. (2002) J. Biol. Chem. 277, 3552–3559. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, J., Ferguson, S. S., Barak, L. S., Menard, L. & Caron, M. G. (1996) J. Biol. Chem. 271, 18302–18305. [DOI] [PubMed] [Google Scholar]
- 30.Goodman, O. B., Jr., Krupnick, J. G., Santini, F., Gurevich, V. V., Penn, R. B., Gagnon, A. W., Keen, J. H. & Benovic, J. L. (1996) Nature 383, 447–450. [DOI] [PubMed] [Google Scholar]
- 31.Oakley, R. H., Laporte, S. A., Holt, J. A., Barak, L. S. & Caron, M. G. (1999) J. Biol. Chem. 274, 32248–32257. [DOI] [PubMed] [Google Scholar]
- 32.Ghanouni, P., Gryczynski, Z., Steenhuis, J. J., Lee, T. W., Farrens, D. L., Lakowicz, J. R. & Kobilka, B. K. (2001) J. Biol. Chem. 276, 24433–24436. [DOI] [PubMed] [Google Scholar]
- 33.Berg, K. A., Maayani, S., Goldfarb, J., Scaramellini, C., Leff, P. & Clarke, W. P. (1998) Mol. Pharmacol. 54, 94–104. [PubMed] [Google Scholar]
- 34.Zhang, J., Ferguson, S. S., Barak, L. S., Bodduluri, S. R., Laporte, S. A., Law, P. Y. & Caron, M. G. (1998) Proc. Natl. Acad. Sci. USA 95, 7157–7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whistler, J. L. & von Zastrow, M. (1998) Proc. Natl. Acad. Sci. USA 95, 9914–9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roettger, B. F., Ghanekar, D., Rao, R., Toledo, C., Yingling, J., Pinon, D. & Miller, L. (1997) J. Mol. Pharmacol. 51, 357–362. [PubMed] [Google Scholar]
- 37.Bhowmick, N., Narayan, P. & Puett, D. (1998) Endocrinology 139, 3185–3192. [DOI] [PubMed] [Google Scholar]
- 38.Gurevich, V. V., Pals-Rylaarsdam, R., Benovic, J. L., Hosey, M. M. & Onorato, J. J. (1997) J. Biol. Chem. 272, 28849–28852. [DOI] [PubMed] [Google Scholar]
- 39.Martini, L., Hastrup, H., Holst, B., Fraile-Ramos, A., Marsh, M. & Schwartz, T. W. (2002) Mol. Pharmacol. 62, 30–37. [DOI] [PubMed] [Google Scholar]
- 40.Watson, C., Chen, G., Irving, P., Way, J., Chen, W. J. & Kenakin, T. (2000) Mol. Pharmacol. 58, 1230–1238. [DOI] [PubMed] [Google Scholar]