Abstract
Epidermal stem cells play a central role in tissue homeostasis, wound repair, tumor initiation, and gene therapy. A major impediment to the purification and molecular characterization of epidermal stem cells is the lack of a quantitative assay for cells capable of long-term repopulation in vivo, such as exists for hematopoietic cells. The tremendous strides made in the characterization and purification of hematopoietic stem cells have been critically dependent on the availability of competitive transplantation assays, because these assays permit the accurate quantitation of long-term repopulating cells in vivo. We have developed an analogous functional assay for epidermal stem cells, and have measured the frequency of functional epidermal stem cells in interfollicular epidermis. These studies indicate that cells capable of long-term reconstitution of a squamous epithelium reside in the interfollicular epidermis. We find that the frequency of these long-term repopulating cells is 1 in 35,000 total epidermal cells, or in the order of 1 in 104 basal epidermal cells, similar to that of hematopoietic stem cells in the bone marrow, and much lower than previously estimated in epidermis. Furthermore, these studies establish a novel functional assay that can be used to validate immunophenotypic markers and enrichment strategies for epidermal stem cells, and to quantify epidermal stem cells in various keratinocyte populations. Thus further studies using this type of assay for epidermis should aid in the progress of cutaneous stem cell-targeted gene therapy, and in more basic studies of epidermal stem cell regulation and differentiation.
There is considerable evidence for a long-term repopulating cell in the interfollicular epidermis (1–4). However, the location of the most primitive stem cell (SC) in terms of production of the interfollicular epidermis is in debate (a follicular vs. interfollicular keratinocyte) (1–8). It is thought by many that cutaneous epithelia contain SCs from a common ectodermal origin that are equipotent, albeit located in different niches (4, 6). One prevailing model of epithelial SC function is that, under steady state conditions, SCs are unipotent (i.e., producing only follicular or only interfollicular epidermis), whereas during regeneration after tissue damage, SCs display multipotency (reviewed in ref. 9). Integrity of the epidermis is maintained by division of cells in the proliferative basal layer to replace differentiated cells in the outermost stratum corneum layer that are continuously being lost. SCs in the basal layer divide to produce, on average, one SC and one transit-amplifying cell (10). The transit-amplifying cells amplify the basal cell population, but are limited to a finite number of cell divisions before they differentiate and are sloughed into the environment (11). SCs are thought to constitute 1–10% of the basal cell population (1, 2, 12–14). However, despite decades of work to characterize the epidermal SC, there still is no standardized quantitative assay to measure it.
We have designed an in vivo assay based on validated functional assays for hematopoietic SCs (15, 16). When competitive long-term repopulating assays combined with a limiting dilution design were used, SC frequency in the bone marrow was determined to be 1 in 10,000 (17, 18). This type of assay has become a standard in hematopoiesis for the analysis of SC markers (19, 20) and for studies of SC differentiation and regulation (21–23). For competitive repopulation assays, one population, the competitor, serves as a standard for repopulating potential. The second population, the test population, is of unknown hematopoietic SC content. Mixtures of competitor and test marrow cells are injected into lethally irradiated mice, and the presence of peripheral blood leukocytes derived from the test cells is assessed 12–20 weeks after transplant by using distinguishable surface markers (e.g., Ly5.1 vs. Ly5.2) (20, 23). Using serial dilutions of test cells, a limiting dilution design is used to measure SC frequency. The validity of limiting dilution in competitive repopulating assays is based on the premise that the assay is sensitive enough to detect the progeny of a single SC. The strength of these assays is the long-term functional nature of the repopulation carried out in vivo that allows the distinction between true SCs vs. shorter-term repopulating cells (transit-amplifying cells in epidermis), and the ability to quantify SCs by competitive limiting dilution analysis.
Our assay for epidermal SCs exploits the ability of dissociated keratinocytes to reform a cornified stratified epithelium when implanted atop the s.c. fascia of immunodeficient mice (24, 25). By studying fluorescent keratinocytes in a competitive repopulating assay at limiting dilutions, we have measured the frequency of functional epidermal SCs in interfollicular epidermis. We find that the frequency of these long-term repopulating cells is surprisingly similar to that of hematopoietic SCs in the bone marrow and is much lower than previously estimated.
Methods
Mice. The GFP-expressing mouse, strain, C57BL/6-TgN(ACTbEGFP)1Osb (The Jackson Laboratory) was used as a source of test keratinocytes. These mice ubiquitously express GFP under the control of the β-actin promoter (26). Keratinocytes from GFP-negative siblings were used as competitor keratinocytes, whereas severe combined immunodeficiency (SCID) mice (Simonsen Laboratories) served as recipients.
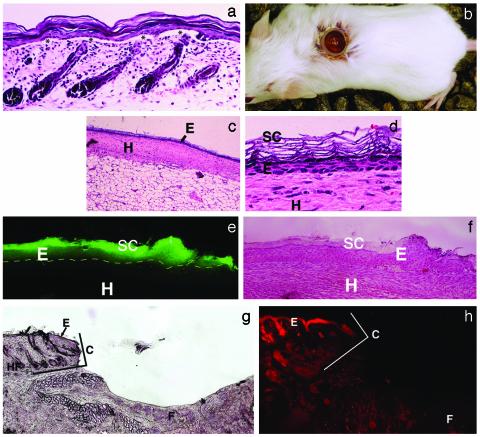
Donor Samples. Donor samples (skin from the trunk of neonatal 3- to 4-day-old mice) were floated in dispase overnight at 4°C, without removal of the s.c. fat. This procedure produces a split along the basal layer (Fig. 1a), such that when the epidermis is scraped off, a full complement of hair follicles can be seen in the remaining dermal portion with some areas where small patches of full thickness epidermis are left behind (not shown). A cell suspension was obtained by placing the epidermal (interfollicular) samples in 0.05% trypsin for 12 min. Varying numbers of GFP-positive keratinocytes were mixed with a fixed number (1 × 106) of GFP-negative keratinocytes. The cells were pelleted, resuspended in 0.1 ml of keratinocyte growth medium with 0.06 mM Ca to form a cell slurry, and then placed into a 6-mm (internal diameter) silicone chamber (Renner, Dannstadt, Germany) on the fascia of recipient mice (25).
Fig. 1.
Reconstitution of murine epidermis on the fascia of SCID mice. (a) Donor cells are from interfollicular epidermis. Note epidermal–dermal split (asterisks), leaving hair follicles in the lower portion of the sample. After scraping, dermis shows a full complement of hair follicles (not shown). (Original magnification, ×40.) (b) Silicone chamber implanted on the fascia of a SCID mouse. (c and d) Grafted murine keratinocytes form an epidermis in 2 weeks. (c) Grafted keratinocytes have formed an epidermis in the chamber at 2 weeks. (Original magnification, × 10.) (d) A high-power view of c. Chamber epidermis shows normal morphology. (Original magnification, ×110.) E, epidermis; SC, stratum corneum; D, dermis; H, host tissue. (e and f) GFP-positive epidermis in a chamber. (e) Grafted GFP-positive keratinocytes have formed an epidermis (E) in the chamber at 2 weeks (fluorescence microscopy). The entire epidermis has positive fluorescence that is particularly bright in the stratum corneum. (f) Hematoxylin staining for detailed morphology (same section). Dotted line indicates dermal/epidermal junction. SC, stratum corneum; D, dermis; H, host tissue. (Original magnification, ×20.) (g and h) Chambers show no ingrowth of host epidermis. (g) There was no evidence of epidermal ingrowth when an empty chamber remained in place for 9 weeks. (Original magnification, ×10.) Normal murine epidermis (E) with hair follicles (HF) is seen on the left. The silicone chamber (location marked C) marks an abrupt end to normal epidermis. In the base of the chamber is the fascia (F) with no signs of viable epidermis. (h) Pancytokeratin–phycoerythrin immunofluorescence of host epidermis adjacent to the chamber, with absence of staining on the fascia inside the chamber. (Original magnification, ×20.)
Chamber Implantation. Mice were anesthetized, and an 0.8-cm area of skin on the back was excised down to the fascia. The chamber, shaped like a top hat with the top cut off (Fig. 1b), was then implanted and sutured in place. After addition of the keratinocytes, tegaderm (3M) was placed over the chamber for 1 week.
Removal of Resultant Epidermis. For long-term repopulating assays, the chambers were harvested at 5–6 weeks or at 9 weeks. Animals were killed, and the chamber epidermis was excised. For limiting dilution studies, a whole mount was prepared, fixed in 2% paraformaldehyde, and examined under an inverted fluorescence microscope (Leica DM IRB inverted microscope).
Statistical Analysis. For limiting dilution assays, the chamber epidermis was scored as positive for test cells if at least one GFP-positive cluster of epidermal cells was detected by microscopy of intact epidermal whole mounts. Statistical analysis was performed by using statistical software programs for limiting dilution analysis [l-calc (Stemsoft, Vancouver) and gemod procedure with a log linear link of a binomial distribution in sas, (SAS Institute, Cary, NC)]. The χ2 statistic was determined to assess the degree of consistency in the data with a Poisson dose–response relationship. A 5% or less type I error was considered to be statistically significant. A significant χ2 test occurs when there is inconsistency in the data distribution.
Keratinocyte Attachment Studies. A total of 106 keratinocytes were implanted into silicone chambers on fascia in vivo, or on collagen IV 0.05 mg/ml (Sigma) in vitro. After 2 h, nonattached cells were removed with three rinses of PBS. Attached cells were then removed with trypsin (0.05% for 12 min) and counted.
Basal Cell Density in the Murine Epidermal Cell Population. Keratinocytes were isolated, fixed in 2% paraformaldehyde, and then permeabilized with 0.01% Triton X-100. Primary antibody was rabbit anti-mouse pAb AF109 against keratin 1 (10 μg/ml; Covance, Berkeley, CA). Secondary antibody was goat anti-rabbit IgG Texas red (Molecular Probes). Nucleated cells were identified with 4′,6-diamidino-2-phenylindole.
Results
Reconstitution/Repopulation of Full-Thickness Murine Epidermis on the Fascia of SCID Mice. After isolation of interfollicular epidermis (Fig. 1a), GFP-positive murine keratinocyte test cells compete against competitor GFP-negative murine keratinocytes to reform an epidermis in vivo. These keratinocytes are mixed and seeded in a chamber on the fascia of a GFP-negative mouse recipient (Fig. 1b). After 2 weeks, a complete epidermis has formed, and chambers are removed at various times after this point to determine, by whole mount microscopy, the presence or absence of GFP-positive cell clusters. We determined that 1,000,000, but not 500,000 or 200,000. keratinocytes are sufficient to consistently produce a complete epidermis in 2 weeks (Fig. 1 c and d). Therefore, in subsequent experiments, a fixed number of competitor GFP-negative keratinocytes (1,000,000) were seeded along with variable numbers of test GFP-positive keratinocytes. When GFP-positive keratinocytes alone were implanted, the resultant epidermis was uniformly GFP positive (Fig. 1 e and f).
To exclude the possibility of ingrowth of keratinocytes from adjacent recipient/host epidermis, implanted chambers were examined for ingrowth over 9 weeks. Ingrowth is unlikely because of the tortuous path required for recipient epidermal keratinocytes to take around the edge of the silicone chamber, and previous studies demonstrated no ingrowth in this type of chamber (25). There was no ingrowth of host epidermis into empty chambers over 9 weeks, as evidenced by lack of a visible epithelial layer (Fig. 1g) and lack of pancytokeratin immunofluorescence (Fig. 1h). In addition, when GFP-negative keratinocytes were implanted on GFP-positive mice, no GFP-positive epidermal cells were seen in the chambers over a 4-week period (data not shown).
We also performed a study to determine how well keratinocytes attached to fascia. We compared the number of keratinocytes that attached to the fascia in a chamber in vivo with the number that attached to collagen IV in a chamber in vitro (a substrate used for rapid murine keratinocyte SC attachment; ref. 27). When 106 cells were implanted in each chamber for 2 h, there was no significant difference between the number of cells that attached to fascia and the number that attached to collagen IV (260,000 ± 61,000 vs. 307,000 ± 56,000, respectively, n = 3). Thus, the fascia appears to be an adequate substrate for keratinocyte attachment.
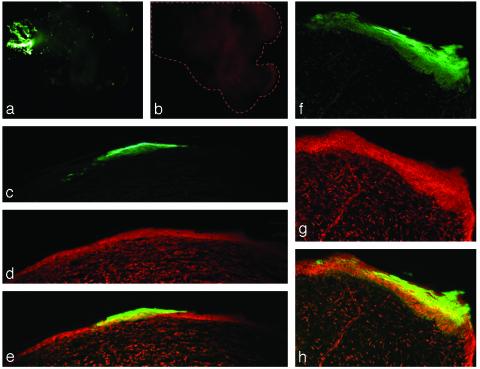
Large GFP-Positive Competitive Repopulation Units Are Detected in the Chamber Epidermis After 5–9 Weeks. Implantation of small numbers of GFP-positive keratinocytes along with 1,000,000 GFP-negative keratinocytes permitted the visualization of individual competitive repopulation units (Fig. 2). At 4–9 weeks, GFP-positive keratinocytes present were part of large epidermal units. A crude estimation of the number of cells in a typical cylindrically shaped unit was in the order of 4,000–6,000 cells. Thus, a single GFP-positive competitive repopulation unit was readily discernable in a background of GFP-negative competitor cells. At early time points (e.g., 4 weeks, Fig. 2a), a few individual GFP-positive stratum corneum cells were seen scattered throughout the reconstituted epidermis, but not at later time points (e.g., 9 weeks). These cells presumably represent more differentiated nonstem keratinocytes that were originally seeded, but that had not yet been sloughed. However, by 4–6 weeks, the earliest time point of examination of these chambers, we did not see small clusters of cells or units only in the upper epidermis. We believe this is because we were looking at too late a time point to observe transit-amplifying cell clones. From these studies we concluded that this method is sufficiently sensitive to detect the progeny of a single SC, a requirement for a valid limiting dilution assay.
Fig. 2.
Competitive repopulating units (CRUs) in grafted epidermis. (a and b) The same GFP-positive CRU surrounded by GFP-negative epidermis (whole mount preparation, viewed with an inverted fluorescent microscope) at 4 weeks. (a) The FITC/GFP channel. At such early time points (4 weeks), some individual GFP-positive cells can also be seen, whereas later (e.g., 9 weeks) they are not seen, presumably because they were differentiated non-SC originally added that are subsequently sloughed off. (b) The red channel as a control for autofluorescence, demonstrating the extent of the piece of tissue (dotted line). (Original magnification, ×2.5.) (c–h) Fluorescence microscopy images of CRUs in grafted epidermis, serially sectioned. (c–e) A single GFP-positive CRU consisting of thousands of GFP-positive cells, surrounded by GFP-negative epidermis (counterstained with propidium iodide). (c) The FITC/GFP channel. (d) The red channel. (e) The overlay of c and d. (f–h) Another such CRU. (Original magnification: c–e ×10; f–h, ×40.)
SC Frequency in Neonatal Murine Epidermis. To determine SC frequency in neonatal murine epidermal cells, dilutions of GFP-positive keratinocytes ranging from 3,250 to 50,000 GFP-positive keratinocytes were implanted. At 5 to 6 weeks, resultant epidermis was graded as positive or negative for GFP-positive competitive repopulation unit(s) by fluorescence microscopy of epidermal whole mounts. The three initial experiments were pooled. Reconstitution was achieved with 12,500 GFP-positive test cells but not with 7,500 or fewer. These results lead to a calculated SC frequency of 1 in 30,000 (95% confidence, 1 in 15,000 to 1 in 60,000) (Table 1). The value of the χ2 test was not statistically significant in Table 1 (P > 0.05), demonstrating internal consistency in our assay. Analysis of the data by using an alternate limiting dilution analysis software program, genmod procedure in sas analysis, gave comparable results. The pooling of the data could be justified by the insignificant differences among the three experiments in the generalized linear model with log link when the experiment was a fixed categorical variable in the genmod analysis.
Table 1. Limiting dilution analysis of SC frequency: Pooled results (experiments 1–3) at 5–6 weeks.
| Positive responses | No. tested | |
|---|---|---|
| Dose of GFP+ keratinocytes | ||
| 50,000 | 3 | 3 |
| 15,000 | 4 | 7 |
| 12,500 | 1 | 1 |
| 7,500 | 0 | 8 |
| 6,250 | 0 | 1 |
| 3,750 | 0 | 9 |
| 3,125 | 0 | 3 |
| Statistical analysis | l-calc | genmod |
| Frequency | 1 in 30,380 | 1 in 30,377 |
| 95% confidence interval | 1 in 60,208 to 1 in 15,329 | 1 in 96,514 to 1 in 18,041 |
| χ2 Pearson | 7.630 | 7.6298 |
| P = 0.2665 | P = 0.2665 | |
| χ2 deviance | 11.601 | 11.601 |
| P = 0.0715 | P = 0.0715 |
A single larger experiment narrowed in on the predicted SC frequency from the initial experiments. The single study (Table 2) was analyzed in a similar fashion, and SC frequency of neonatal murine epidermal cells was 1 in 26,000 (95% confidence limits, 1 in 14,000 to 1 in 51,000), in close agreement with the results of the first pooled study.
Table 2. Limiting dilution analysis of SC frequency: Experiment 4 at 5–6 weeks.
| Positive responses | No. tested | |
|---|---|---|
| Dose of GFP+ keratinocytes | ||
| 30,000 | 5 | 6 |
| 15,000 | 4 | 8 |
| 7,500 | 0 | 8 |
| Statistical analysis | l-calc | genmod |
| Frequency | 1 in 26,479 | 1 in 26,455 |
| 95% confidence interval | 1 in 50,914 to 1 in 13,771 | 1 in 76,453 to 1 in 16,013 |
| χ2 Pearson | 3.432 | 3.4317 |
| P = 0.1798 | P = 0.1798 | |
| χ2 deviance | 5.425 | 5.4255 |
| P = 0.0664 | P = 0.0664 |
Finally, to determine that the assay was measuring a true long-term repopulating cell, rather than a shorter-term transit-amplifying cell, we performed the same assay at 9 weeks. SC frequency after 9 weeks of reconstitution was 1 in 41,000 (95% confidence limits, 1 in 72,000 to 1 in 23,000) (Table 3). The P value for difference in SC frequency between weeks 6 and 9 was not significant (P = 0.32 for l-calc, P = 0.36 for genmod). Furthermore, the data from Table 2 (5–6 weeks) and Table 3 (9 weeks) were combined, resulting in a SC frequency of 1 in 35,000. Combining the data resulted in an insignificant χ2 test, confirming the consistency of the data distribution between 5 and 9 weeks.
Table 3. Limiting dilution analysis of SC frequency: 9 weeks.
| Positive responses | No. tested | |
|---|---|---|
| Dose of GFP+ keratinocytes | ||
| 60,000 | 6 | 7 |
| 30,000 | 3 | 7 |
| 15,000 | 3 | 10 |
| 7,500 | 1 | 7 |
| Statistical analysis | l-calc | genmod |
| Frequency | 1 in 40,909 | 1 in 40,917 |
| 95% confidence interval | 1 in 71,701 to 1 in 23,340 | 1 in 93,284 to 1 in 26,205 |
| χ2 Pearson | 0.570 | 0.5699 |
| P = 0.9033 | P = 0.8974 | |
| χ2 deviance | 0.606 | 0.6060 |
| P = 0.8951 | P = 0.8951 |
Basal Cell Density in the Murine Epidermal Cell Population. Previous estimates of epidermal SC frequency used the basal cell as the denominator (1, 2, 12–14). To compare the above results to prior studies, we performed a crude estimate of the basal cell density of neonatal murine epidermis, by determining the proportion of nucleated keratinocytes (4′,6-diamidino-2-phenylindole positive) that were keratin 1 negative. Keratin 1 protein is found predominantly in suprabasal cells, although occasional basal cells may be positive (28). By this method, we found the basal cell density to be 47 ± 4%. Thus, if 1 in 26,000 to 1 in 40,000 keratinocytes is a SC, and 47% are basal cells, then in the order of 0.01% of basal cells are SCs, a frequency similar to that seen in nucleated bone marrow cells, and much less than previously estimated for keratinocyte SCs by other methods.
Discussion
Previous methods of SC analysis in epidermis include short-term in vitro clonogenic assays of human keratinocytes and in vivo studies of label-retaining cells in mice. Clonogenic assays of human keratinocytes in vitro may overestimate SC number, because not all of the cells that generate large colonies may be capable of long-term epidermal regeneration (6). When mice are serially injected with tritiated thymidine or BrdUrd, almost 100% of the basal cells become labeled. After 30–60 days, there is a pool of cells that still retains label; it is proposed that, because label-retaining cells must be relatively quiescent, they may represent murine epidermal SCs (12). An inherent limitation of these two types of assays is that they do not directly measure the ability of the cells being studied to functionally recreate an in vivo epidermis.
One important question regarding any functional assay for SCs is what duration of repopulation is required to distinguish the true epidermal SC from more short-term repopulating cells. In the epidermis, the short-term repopulating cells are the transit-amplifying cells. In murine epidermis, transit time from basal layer to granular layer has been estimated to be 7–10 days (29). The cell cycle time in mammalian epidermis has been estimated to be 4–5 days (30, 31). Transit-amplifying cells are expected to go through approximately three divisions (32) before terminally differentiating. However, the SC is a cell considered to be out of step with this general program. There are several lines of evidence suggesting that 30 days of epidermal turnover is an adequate interval for assessing long-term repopulation of skin. In a study examining loss of tritiated thymidine over time in epidermis, 35% of basal cells retained >45 grains of tritiated thymidine at 1 h, but only 0.2% retained >45 grains of label at 30 days (33). Because the cell cycle time of proliferative cells in adult mouse ear is ≈4.8 days and may be shorter in a younger growing animal, such cells retaining label at 30 days were cycling markedly out of phase with respect to the rest of the epithelial cells, suggesting that these may be SCs (12). The number of these label-retaining cells detected at 30 days and at 72 days, was similar, whereas all cells with <45 grains continued to lose label over time (33), suggesting that the most primitive SC may be detected at 30 days.
In the case of murine hematopoietic SCs, the intervals used to assess long-term repopulation range from 12 to 20 weeks after transplantation (34). Assays performed at early time points are thought to overestimate the frequency of SCs, as they would additionally measure the progeny of some short-term repopulating cells. Because the hierarchy of epidermal SC differentiation is poorly understood, we decided to assess long-term repopulation first at 35–42 days (5–6 weeks) and then at 9 weeks. In our studies, the number of repopulating cells detected did not decrease significantly at 9 weeks vs. 5–6 weeks, strongly suggesting that we are measuring the most primitive SC at 5–6 weeks.
The assay, as used in this study, tests only for SCs derived from interfollicular epidermis, and confirms that long-term repopulating cells do in fact reside in this location. It is possible that there may be more numerous and/or more primitive (capable of longer-term repopulation) SCs located in the follicular bulge (7). Further studies using follicle-derived keratinocytes vs. interfollicular keratinocytes as test cells in this assay will help clarify these issues.
The assay, as currently designed, measures only the ability of cells to form an epidermis, and hence does not test the pluripotency of these SCs, i.e., their ability to form hair follicles and sebaceous glands. Under appropriate conditions, interfollicular epithelium has been shown to regenerate hair and sebocyte lineages (35–37). Only after future studies using this assay to identify the most effective marker(s) for long-term repopulating cells (see below) will we finally be able to test for the multipotency of the long-term repopulating cell that we are measuring in this assay.
Previous estimates of SC frequency in the epidermis, based on quantitative radiobiological experiments, are in the order of 10% of basal cells (ref. 38; reviewed in ref. 6). Another study suggests that only 4% of the basal cells are SCs (27). However, as discussed above, only 0.2% of cells continue to retain large amounts of label without significant loss over 30–72 days (33). Because our studies indicate that 47% of neonatal murine epidermal cells are basal cells, and 1 in 26,000 to 1 in 40,000 epidermal cells is a SC, in the order of 0.01% of basal cells are SCs. It is notable that the frequency of epidermal SCs in this assay is similar to the frequency of hematopoietic SCs in the marrow (0.01%). One potential explanation for the much lower than expected frequency of repopulating units measured in this assay is that the fascia might be a suboptimal environment for epidermal SCs. The fact that the murine keratinocytes adhere to fascia as efficiently as to collagen IV is somewhat reassuring. However, it is still theoretically possible that epidermal SCs proliferate less efficiently on fascia than in their natural environment. In this unlikely to be the case, this functional assay would still permit the accurate measurement of the relative repopulating abilities of keratinocytes from different sources.
The concept of an epidermal proliferation unit (reviewed in ref. 39) is that of a SC and ≈10 surrounding basal cells supplying the epidermal cells above (<50 cells in total). This structure appears to be much smaller than the structures observed in our studies and by others (see refs. 3 and 40). In each of these studies, a much larger unit containing hundreds to thousands of cells appears to be supplied by a single cell. In another study, large epidermal units were observed with a hyperproliferative state (41). To reserve the term epidermal proliferation unit for the organizational structure observed by Potten (42), we have termed the units produced by the long-term repopulating SCs in this assay “competitive repopulation units,” a standard term used for hematopoietic SCs.
This study uses competitve repopulation combined with limiting dilution analysis to measure an absolute frequency. However, this type of assay may also be used at nonlimiting dilution to assess the efficiency of various methods of SC isolation. This type of study measures the “fold improvement” in the repopulating ability of purified test populations vs. unsorted test cells (20). A number of methods have been proposed to enrich for epidermal SCs, but controversy exists regarding the best epidermal SC marker (10). Methods that isolate populations enriched in epidermal SCs include (i) highest levels of β1 integrin (43); (ii) high levels of α6 integrin in combination with the lack of a transferrin receptor (44); and (iii) a Hoechst and propidium iodide dye technique combined with specifically defined gating (45). This assay provides an in vivo functional assay by which to examine the effectiveness of these isolation techniques in identifying true long-term repopulating cells. Based on the lessons learned concerning hematopoietic SCs, we anticipate that the immunophenotype of epidermal SCs will prove to be complex, and require a number of positive and negative selection markers, which can then be validated by this assay.
In summary, this novel assay has confirmed the existence of a long-term repopulating cell in the interfollicular epidermis, the frequency of which is 1 in 26,000 to 1 in 40,000. This study has established a quantitative in vivo assay for keratinocyte SCs, analogous to that used for hematopoietic SCs. This in vivo functional assay for epidermal SCs, is an important step for SC quantitation of various keratinocyte populations (e.g., aging, development, and disease), for analyzing putative markers for the epidermal SC, for cutaneous SC-targeted gene therapy, and for more basic studies of epidermal SC regulation and differentiation.
Acknowledgments
We thank Daniel Bickle, M.D., for helpful advice and comments. This work was supported by National Institutes of Health Grants AG20372-01 and AR48667-02 (to R.G.), DK48642 (to H.J.L.), and ES8061 (to J.E.C.), a Department of Veterans Affairs Merit Review Program Award (to R.G.), and an Ellison Senior Scholar Award (to J.E.C.).
Abbreviation: SC, stem cell.
References
- 1.Morris, R. J., Fischer, S. M. & Slaga, T. J. (1985) J. Invest. Dermatol. 84, 277–281. [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie, I. C. & Bickenbach, J. R. (1985) Cell Tissue Res. 242, 551–556. [DOI] [PubMed] [Google Scholar]
- 3.Ghazizadeh, S. & Taichman, L. B. (2001) EMBO J. 20, 1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niemann, C. & Watt, F. M. (2002) Trends Cell Biol. 12, 185–192. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs, E. & Raghavan, S. (2002) Nat. Rev. Genet. 3, 199–209. [DOI] [PubMed] [Google Scholar]
- 6.Cotsarelis, G., Kaur, P., Dhouailly, D., Hengge, U. & Bickenbach, J. (1999) Exp. Dermatol. 8, 80–88. [DOI] [PubMed] [Google Scholar]
- 7.Taylor, G., Lehrer, M. S., Jensen, P. J., Sun, T. T. & Lavker, R. M. (2000) Cell 102, 451–461. [DOI] [PubMed] [Google Scholar]
- 8.Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. & Barrandon, Y. (2001) Cell 104, 233–245. [DOI] [PubMed] [Google Scholar]
- 9.Slack, J. M. (2000) Science 287, 1431–1433. [DOI] [PubMed] [Google Scholar]
- 10.Lavker, R. M. & Sun, T. T. (2000) Proc. Natl. Acad. Sci. USA 97, 13473–13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potten, C. S. (1981) Int. Rev. Cytol. 69, 271–318. [DOI] [PubMed] [Google Scholar]
- 12.Bickenbach, J. R., McCutecheon, J. & Mackenzie, I. C. (1986) Cell Tissue Kinet. 19, 325–333. [DOI] [PubMed] [Google Scholar]
- 13.Morris, R. J. & Potten, C. S. (1994) Cell Prolif. 27, 279–289. [DOI] [PubMed] [Google Scholar]
- 14.Heenen, M. & Galand, P. (1997) Dermatology 194, 313–317. [DOI] [PubMed] [Google Scholar]
- 15.Harrison, D. E. (1980) Blood 55, 77–81. [PubMed] [Google Scholar]
- 16.Chang, H., Jensen, L. A., Quesenberry, P. & Bertoncello, I. (2000) Exp. Hematol. 28, 743–752. [DOI] [PubMed] [Google Scholar]
- 17.Szilvassy, S. J., Humphries, R. K., Lansdorp, P. M., Eaves, A. C. & Eaves, C. J. (1990) Proc. Natl. Acad. Sci. USA 87, 8736–8740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taswell, C. (1981) J. Immunol. 126, 1614–1619. [PubMed] [Google Scholar]
- 19.Weissman, I. L. (2002) Immunol. Rev. 185, 159–174. [DOI] [PubMed] [Google Scholar]
- 20.Spangrude, G. J., Heimfeld, S. & Weissman, I. L. (1988) Science 241, 58–62. [DOI] [PubMed] [Google Scholar]
- 21.Mikkola, H. K., Klintman, J., Yang, H., Hock, H., Schlaeger, T. M., Fujiwara, Y. & Orkin, S. H. (2003) Nature 421, 547–551. [DOI] [PubMed] [Google Scholar]
- 22.Smith, L. G., Weissman, I. L. & Heimfeld, S. (1991) Proc. Natl. Acad. Sci. USA 88, 2788–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park, I. K., Qian, D., Kiel, M., Becker, M. W., Pihalja, M., Weissman, I. L., Morrison, S. J. & Clarke, M. F. (2003) Nature 423, 302–305. [DOI] [PubMed] [Google Scholar]
- 24.Fusenig, N. E., Amer, S. M., Boukamp, P. & Worst, P. K. (1978) Bull. Cancer 65, 271–279. [PubMed] [Google Scholar]
- 25.Wang, C. K., Nelson, C. F., Brinkman, A. M., Miller, A. C. & Hoeffler, W. K. (2000) J. Invest. Dermatol. 114, 674–680. [DOI] [PubMed] [Google Scholar]
- 26.Hadjantonakis, A. K., Gertsenstein, M., Ikawa, M., Okabe, M. & Nagy, A. (1998) Mech. Dev. 76, 79–90. [DOI] [PubMed] [Google Scholar]
- 27.Bickenbach, J. R. & Chism, E. (1998) Exp. Cell Res. 244, 184–195. [DOI] [PubMed] [Google Scholar]
- 28.Schweizer, J., Kinjo, M., Furstenberger, G. & Winter, H. (1984) Cell 37, 159–170. [DOI] [PubMed] [Google Scholar]
- 29.Potten, C. S. (1975) Br. J. Dermatol. 93, 649–658. [DOI] [PubMed] [Google Scholar]
- 30.Gelfant, S. (1982) J. Invest. Dermatol. 78, 296–299. [DOI] [PubMed] [Google Scholar]
- 31.Hegazy, M. A. & Fowler, J. F. (1973) Cell Tissue Kinet. 6, 17–33. [DOI] [PubMed] [Google Scholar]
- 32.Watt, F. M. (1998) Philos. Trans. R. Soc. London Ser. B 353, 831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bickenbach, J. R. (1981) J. Dent. Res. 60, 1611–1620. [DOI] [PubMed] [Google Scholar]
- 34.Zhong, R. K., Astle, C. M. & Harrison, D. E. (1996) J. Immunol. 157, 138–145. [PubMed] [Google Scholar]
- 35.Reynolds, A. J. & Jahoda, C. A. (1992) Development (Cambridge, U.K.) 115, 587–593. [DOI] [PubMed] [Google Scholar]
- 36.Ferraris, C., Bernard, B. A. & Dhouailly, D. (1997) Int. J. Dev. Biol. 41, 491–498. [PubMed] [Google Scholar]
- 37.Ferraris, C., Chevalier, G., Favier, B., Jahoda, C. A. & Dhouailly, D. (2000) Development (Cambridge, U.K.) 127, 5487–5495. [DOI] [PubMed] [Google Scholar]
- 38.Potten, C. S. & Hendry, J. H. (1973) Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 24, 537–540. [DOI] [PubMed] [Google Scholar]
- 39.Potten, C. S. & Booth, C. (2002) J. Invest. Dermatol. 119, 888–899. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, W., Remenyik, E., Zelterman, D., Brash, D. E. & Wikonkal, N. M. (2001) Proc. Natl. Acad. Sci. USA 98, 13948–13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mackenzie, I. C. (1997) J. Invest. Dermatol. 109, 377–383. [DOI] [PubMed] [Google Scholar]
- 42.Potten, C. S. (1974) Cell Tissue Kinet. 7, 77–88. [DOI] [PubMed] [Google Scholar]
- 43.Jones, P. H. & Watt, F. M. (1993) Cell 73, 713–724. [DOI] [PubMed] [Google Scholar]
- 44.Tani, H., Morris, R. J. & Kaur, P. (2000) Proc. Natl. Acad. Sci. USA 97, 10960–10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunnwald, M., Tomanek-Chalkley, A., Alexandrunas, D., Fishbaugh, J. & Bickenbach, J. R. (2001) Exp. Dermatol. 10, 45–54. [DOI] [PubMed] [Google Scholar]