Abstract
Fossil Foraminifera appear in the Early Cambrian, at about the same time as the first skeletonized metazoans. However, due to the inadequate preservation of early unilocular (single-chambered) foraminiferal tests and difficulties in their identification, the evolution of early foraminifers is poorly understood. By using molecular data from a wide range of extant naked and testate unilocular species, we demonstrate that a large radiation of nonfossilized unilocular Foraminifera preceded the diversification of multilocular lineages during the Carboniferous. Within this radiation, similar test morphologies and wall types developed several times independently. Our findings indicate that the early Foraminifera were an important component of Neoproterozoic protistan community, whose ecological complexity was probably much higher than has been generally accepted.
The geological record of a group of organisms is marked by the appearance of its fossilized remains, yet the true evolutionary history of the group may include a significant nonfossilized period. Molecular data provide an important tool with which to investigate this otherwise cryptic period, by permitting inference of the phylogeny of extant species that may be related to ancestral forms, and by providing molecular clocks by which to estimate their divergence times. For example, molecular phylogenetic studies suggest that the Cambrian explosion of animals was preceded by a long period of divergence of nonskeletonized ancestors (1, 2). Precambrian origins were also proposed for plants and fungi based on a multigene study (3). In contrast, the early history of the main protozoan groups remains uncertain at the molecular level.
The Foraminifera represent one of the most ecologically important groups of marine heterotrophic protists (4). Because of their excellent fossil record, the evolutionary history is well known for biomineralized foraminiferal lineages, and many of these are key indices in biostratigraphic, paleoceanographic, and paleoclimatic reconstructions. Detailed knowledge of foraminiferal evolution, however, is largely limited to agglutinated and calcareous multilocular species, which radiated during the Carboniferous (5, 6). Comparatively little is known about the evolution of noncalcareous unilocular Foraminifera, whose thecate (organic-walled) or agglutinated tests are rarely encountered in the fossil record since the Early Cambrian (7, 8). There is even less geological information regarding “naked” species lacking tests, which may have played a pivotal role in the evolution of the group.
Traditionally, the evolution of early Foraminifera is viewed as a gradual process of change in the composition and structure of the test wall, starting from simple soft-walled thecate unilocular forms that developed an agglutinated wall and later evolved into multilocular forms (9). It has been proposed that the first agglutinated Foraminifera were either globular or tubular species that progressively evolved by development of a proloculus (initial chamber) followed by a rectilinear or coiled tubular chamber (10). Alternatively, based on a literal interpretation of the sparse Cambrian foraminiferal fossil record (8, 11) and the recent identification of a proloculus in the early foraminiferan, Platysolenites antiquissimus, it has been suggested that all Foraminifera evolved from Platysolenites, either by losing the proloculus to become globular or tubular, or by the development of spiral growth (12). The evolution of spiral tests led to the formation of internal septae through the development of constrictions in the spiral tubular chamber and hence the appearance of multilocular forms.
Because of their poor preservation and the difficulties involved in their identification, the unilocular noncalcareous foraminifers are largely ignored in paleontological studies. In a previous study, we used molecular data to reveal the presence of naked foraminifers, perhaps resembling those that lived before the first skeletonized species appeared (13, 14). Here, we investigate the molecular phylogeny of naked, thecate, and agglutinated unilocular species to identify the major steps in the evolution of early Foraminifera.
Materials and Methods
Specimen Collection and DNA Sequencing. Sequence data were obtained for 53 species and 18 undetermined morphotypes of unilocular Foraminifera, and 21 multilocular species. Most of unilocular foraminifers were collected from coastal (McMurdo Sound) and deep-sea (Weddell Sea) Antarctic localities, and from Arctic (Svalbard) and Scandinavian fjords (Oslofjord, Kosterfjord), where unilocular species are particularly abundant. Additionally, eight sequences were obtained from freshwater environmental samples collected in Switzerland and the United States. Detailed information on collection localities is given in Table 1, which is published as supporting information on the PNAS web site, www.pnas.org. DNA was extracted from freshly collected specimens by using either the guanidine lysis buffer or a DNeasy Plant Minikit (Qiagen, Basel). A fragment of the small subunit (SSU) rRNA gene was amplified by using foraminiferal-specific primers s14F3 (5′-ACG CA(AC) GTG TGA AAC TTG) and sB (5′-TGA TCC TTC TGC AGG TTC ACC TAC). PCR amplifications, cloning, and sequencing were done as described (15). Several clones were sequenced for each isolate, and, whenever it was possible, several isolates were sequenced for each morphospecies.
Phylogenetic Analyses. The 79 SSU rRNA gene sequences of unilocular foraminifers were manually aligned with sequences from 21 multilocular foraminifers by using seaview software (16). We analyzed 552 unambiguously aligned positions. Evolutionary trees were inferred by using the neighbor-joining (NJ) and the maximum likelihood (ML) methods. Distances were corrected by using the K2P model of substitution (17) for NJ analyses, and the F84 model of substitution (18) for ML analyses. The reliability of internal branches was assessed by using the bootstrap method (19) with 1,000 replicates for NJ analyses and 100 replicates for ML analyses. The phylo win program (16) was used for distance computations, tree building, and bootstrapping. Additionally, ML analyses were performed with paup* (20), by using the general time reversible (GTR) model of substitution, taking into account a proportion of invariant sites, and a gamma-shaped distribution of rates of substitution among sites, with eight rate categories (21, 22). All necessary parameters were estimated from the data by using modeltest (23). Starting trees were obtained via NJ and swapped with the tree bisection-reconnection algorithm.
Tree Calibration. Relative rate tests were performed with rrtree (24) to exclude all lineages or individual sequences that display significantly higher rates of substitution. Rate homogeneity among the remaining sequences was then evaluated by using a likelihood ratio test (25). Significance was assessed by comparing D = –2 LR (where LR is the difference between the Log likelihood of the tree, with and without enforcing a molecular clock) with a χ2 distribution (with n-2° of freedom, where n is the number of taxa). The log likelihood of both trees was calculated with paup* (20), and all necessary parameters were estimated from the data by using modeltest (23). Based on calibration dates corresponding to major foraminiferal radiation events recorded in the fossil record, we calculated the mean rate of substitution within the clade of multilocular species, and then applied this rate to the rest of the tree to obtain an estimate for the timing of the initial radiation of foraminiferal lineages and the subsequent radiation leading to the divergence of multilocular species.
Results and Discussion
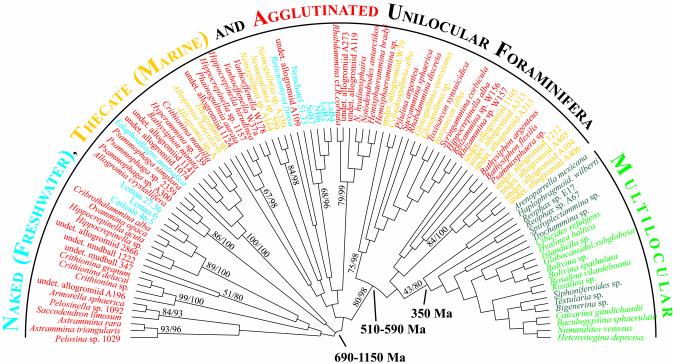
Phylogenetic analyses of our data using distance and ML methods show a large radiation of naked and unilocular foraminifers preceding the divergence of multilocular species (Fig. 1). At least 13 lineages can be identified within this radiation (see ref. 26 for taxonomic details). Most of them are monotypic and are composed of single or related genera. The relationships among the various lineages are difficult to resolve. A distinctive radiation, supported by high bootstrap values (80–98%), includes a few unilocular lineages characterized by a wide variety of morphotypes, as well as the clade that contains all multilocular species having agglutinated (Textulariida) and calcareous perforate (Rotaliida) tests.
Fig. 1.
Phylogenetic relationships among early Foraminifera inferred from partial small subunit rRNA gene sequences. The various types of test are highlighted with different colors. Among multilocular foraminiferans, the Rotaliida are marked in green, whereas the Textulariida are marked in dark green. The tree was calibrated according to the fossil radiation of multilocular Foraminifera (350 Ma). The time ranges for the initial radiation of unilocular species, as well as the radiation leading to the divergence of multilocular species, are indicated. The topology shown was obtained with the ML method, by using the F84 substitution model. Because the exact position of the root remains unresolved, the tree is drawn with a basal trichotomy. However, the placement of the root does not influence the general topology of the tree and has little influence on the tree calibration. The bootstrap support values for the main lineages in ML and NJ analyses are indicated at internal nodes. The presented tree does not differ markedly from that obtained with ML analysis performed by using the GTR substitution model, taking into account a proportion of invariant sites and a gamma-shaped distribution of rates of substitution among sites, with eight rate categories. The only differences relate to the relative branching order of the unilocular lineages and, most particularly, to the position of the monogeneric groups (Nemogullmia, Reticulomyxa, Tinogullmia, and Vanhoeffenella).
A striking feature of our data is the lack of evidence for a progressive increase in the complexity of the foraminiferal test (in terms of both its wall structure and its gross morphology) as had been suggested by the classical views of the early evolution of the Foraminifera (9, 10). The naked species (Reticulomyxa filosa) examined here, as well as the putative naked species detected in freshwater environmental DNA samples, branch in several independent clades. The existence of these clades does not precede the evolution of testate lineages, and such naked organisms probably lost their tests secondarily, for example, as an adaptation to the freshwater environment (14). According to our data, the evolution of early Foraminifera consisted of a series of tentative experiments to develop a test by using various materials and construction methods. There is no clear separation between thecate and agglutinated taxa, and several lineages include both types of wall. Moreover, similar morphotypes developed independently in different lineages (see the positions of Hippocrepinella, Bathysiphon, and Crithionina in Fig. 1). Morphological variations in some lineages by far exceed the traditional morphology-based taxonomy. For example, the Antarctic notodendrodids comprise several morphotypes, including spherical, tubular, and arborescent forms, some of them present together in a single species (27). This evolutionary plasticity among early Foraminifera makes their present morphology-based classification of limited value. We conclude that the thecate or agglutinated walls in unilocular Foraminifera are convergent features, and that the simple evolutionary progression from one to the other, as envisaged by earlier authors (9, 10), did not occur.
Given the wide variability of test structures, the only obvious common character of early Foraminifera is the presence of web-like, granular pseudopodia (granuloreticulopodia). These complex pseudopodia are likely derived from much simpler filopodia, as suggested by the close relationship between the Foraminifera and the Cercozoa inferred from actin-based phylogenies (28) and novel polyubiquitin structure (29). Indeed, the early Foraminifera may have evolved from testate cercozoans, such as Gromia oviformis, which appears as their sister group in molecular phylogenies (30). The distinguishing features of reticulopodia, such as rapid bidirectional movement of intracellular organelles and plasma membrane surface domains, and development of extensive networks, provided early Foraminifera with a greatly enhanced ability to gather and manipulate particles and to construct various types of test (31). The development of reticulopodia, and the subsequent building of the test, were crucial for the initial diversification of the group, providing the Foraminifera with shelter from predation and adverse environmental conditions, as well as with a compartment in which to store food and to protect juveniles (32).
The precise dating of the divergence of the Foraminifera from their cercozoan ancestor is difficult because of the accelerated rates of SSU rRNA gene evolution in the foraminiferal stem lineage (33). Therefore, we estimate the beginning of foraminiferan radiation based on local molecular clocks. We calibrate our molecular tree by using the Carboniferous diversification of multilocular Foraminifera, ≈350 mega-annum (Ma) (6). This is a very conservative calibration because the earliest example of an indisputable multilocular foraminiferan test in the fossil record is a uniserial Reophax from the Middle Ordovician, ≈460 Ma (34). Based on this fossil calibration, we calculate that the rate of substitution within the clade of multilocular species averages 0.03 substitutions/1,000 sites/million years. By removing the lineages that deviate significantly from this rate, we clock the tree and estimate that the radiation of early Foraminifera occurred between 690 and 1,150 Ma (Fig. 1). This time is congruent with the Neoproterozoic origins of the majority of eukaryotic clades suggested by both fossil (35) and molecular (3) data. It is also in agreement with the reassignment of the vase-shaped microfossils found widely in Neoproterozoic rocks to an extant group of filose amoebae (36), which are related to the Foraminifera according to molecular studies (28, 29).
According to our data, an important event occurred within the radiation of early Foraminifera, between 510 and 590 Ma (Fig. 1). This event is evidenced in the molecular tree by a rapidly evolving stem lineage leading to the later divergence of a multilocular clade and a few unilocular lineages. Interestingly, the group of unilocular lineages includes such diverse morphotypes as the xenophyophore Syringammina, the astrorhizids Rhabdammina, Saccammina, and Psammosphaera, as well as the allogromiid Gloiogullmia. Many of these are typical bathyal forms today. The wide morphological variability and close genetic relationships among species belonging to this radiation indicate a very rapid tempo of morphological evolution, in contrast to other lineages that are, in general, morphologically homogenous. A similar increase in diversity is observed in Cambrian acritarchs and other protistan microfossils, coincident with the radiation of marine invertebrates (37).
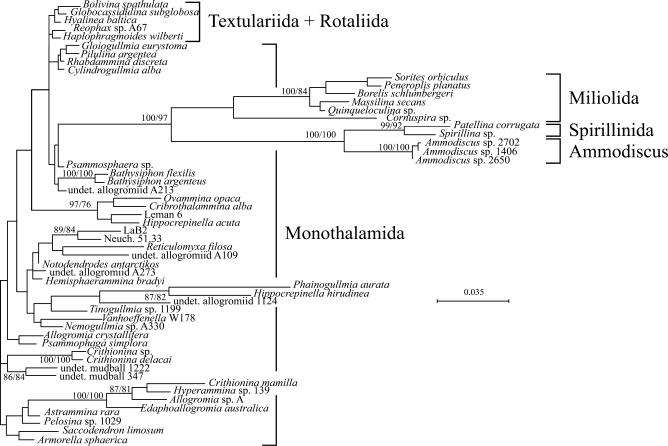
By combining molecular and fossil data, we obtain new insight into the evolution of early Foraminifera (Fig. 2). The Cambrian appearance of fossil foraminiferans seems to be preceded by a large radiation of nonfossilized naked and unilocular species, which diverged from a cercozoan ancestor in the Neoproterozoic. This radiation gave rise to a wide variety of morphological forms that presently colonize all types of marine and freshwater environments; we classify these as Monothalamida, with reference to an earlier taxonomic scheme (38). In the Cambrian, another distinct radiation is observed, leading to the divergence of a few highly variable unilocular lineages and the clade of multilocular species, including agglutinated Textulariida and calcareous Rotaliida (Fig. 2). Another group of calcareous Foraminifera belonging to the order Miliolida, probably diverged from an Ammodiscus-like lineage, which appeared in the Early Cambrian (7, 8). According to our data (Fig. 2), members of the Ammodiscus lineage also gave rise to the calcareous order Spirillinida, in agreement with a recent morphotaxonomic study (39). The independent origins of multilocular calcareous rotaliids and miliolids are consistent with the distinctive modes of biomineralization in these groups (40).
Fig. 2.
Phylogenetic relationships among 55 Foraminifera inferred from partial small subunit rDNA sequences, including representatives of all groups shown in Fig. 1, as well as 3 members of the genus Ammodiscus, 7 members of the order Miliolida, and 2 members of the order Spirillinida. Because of the high divergence of the SSU rDNA sequences in the three latter groups, 500 unambiguously aligned positions were kept in phylogenetic analyses for this dataset. The topology shown was obtained with the ML method by using the F84 substitution model. Because the exact position of the root is yet unclear, the tree is drawn with a basal trichotomy. Representatives of the genus Ammodiscus and the orders Miliolida and Spirillinida form a clearly monophyletic group, but their placement as a sister group to Psammosphaera sp. is not supported. Due to the reduced number of analyzed positions, the resolution among the other groups of Foraminifera is weaker than in Fig. 1. The bootstrap support values >80% for NJ and ML analyses are indicated at internal nodes.
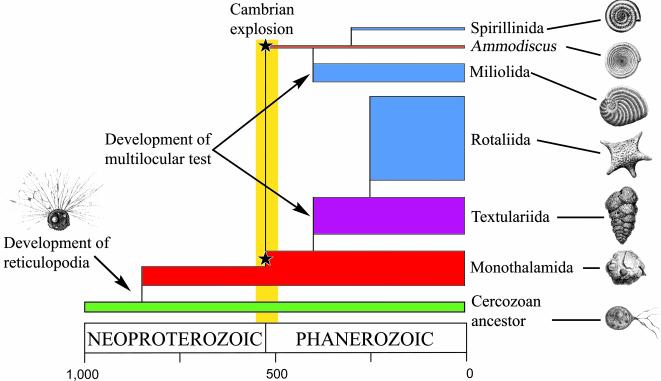
Our data implicate, for the first time, the Foraminifera as an important component of Neoproterozoic protistan communities (Fig. 3). As a result, some Neoproterozoic microfossils (36) or trace fossils (41) may require reevaluation to verify whether or not they represent unilocular foraminiferans. For example, the interpretation of some Upper Vendian microfossils as agglutinated foraminifers (42) seems accurate in view of our study.
Fig. 3.
Time scale of early foraminiferal evolution based on combined molecular and fossil data, highlighting the development of reticulopodia at the origin of the group, and the independent development of a multilocular test in the lineages leading to Textulariida + Rotaliida and Spirillinida + Miliolida. Only the taxonomic groups for which molecular data exist are illustrated. The height of each rectangle is proportional to the number of recognized families in the clade, or to the number of different genetic lineages in the case of Monothalamida. Stars indicate the fossil appearance of some unilocular lineages.
By analogy to the diversity of modern unilocular Foraminifera, we speculate that Neoproterozoic ecological complexity was higher than has been generally accepted. Modern foraminifers acquire nutrients through an exceedingly broad range of trophic strategies, ranging from osmotrophy (43) to various holotrophic mechanisms (44). The radiation of foraminiferal trophic strategies would probably not be possible without a rich microbial eukaryotic community, offering a wide variety of trophic resources that could be exploited by early foraminifers. Species with carnivorous habits similar to those of modern larger agglutinated Foraminifera (45) might even have helped shape early animal evolution by forcing prey organisms to adopt various avoidance or resistance modalities. It may not be coincidental that one of the most diverse assemblages of modern unilocular Foraminifera is found in the macrofauna-poor benthic community of Explorers Cove, Antarctica (15, 46). This cold and relatively undisturbed coastal setting, characterized by low animal diversity and seasonally pulsed planktonic productivity (47), may serve as a useful model of the Neoproterozoic marine benthic ecosystem.
Our data also permit the identification of those unilocular foraminiferan species that are most closely related to multilocular lineages. This information represents an important first step in the selection of model systems for cell and molecular studies of the architectural basis for multilocularity in this group. The Cambrian radiation, leading to the appearance of multichambered tests, may have been driven by changes in foraminiferal cell biology. For example, multilocularity results in cytoplasmic compartmentalization, a trait that Foraminifera have exploited so as to diversify physiologically. Thus, certain multilocular species use their inner chambers to house symbionts whereas the outer (younger) chambers are used to elaborate on digestive or reproductive functions (48). In this sense, multilocularity parallels tissue-level organization in metazoans. Although much progress has been made in identifying the genetic basis of metazoan architecture, comparatively little is known about protistan architectural genes. With these new data on unilocular Foraminifera, we can now proceed with experimental work to test various hypotheses regarding the adaptive significance of multilocularity in this group.
Supplementary Material
Acknowledgments
We thank I. Bolivar, N. Butterfield, T. Cavalier-Smith, G. Gudmundsson, M. Kaminski, and L. Zaninetti for helpful discussions and comments on the manuscript; H. Giles, J. Guiard, and S. Mérolle for technical assistance; A. Brandt, B. Hilbig, D. Fütterer, and the captain and crew of RV Polarstern [Antarctic Benthic Deep-Sea Biodiversity (ANDEEP) 2 cruise] and M. Hald, S. Korsun, and the captain and crew of RV Jan Mayen (Svalbard cruise), as well as S. Goldstein and J.-P. Debenay for their help in collecting coastal North American and European Foraminifera. We are indebted to D. Coons, N. W. Pollock, S. Alexander, P. Forte, J. Bernhard, G. Gwardschaladse, and S. Hanes for diving and field assistance, and the staffs of the National Science Foundation Office of Polar Programs, Antarctic Support Associates, and Squadron VXE-6 for Antarctic logistic support. The Wadsworth Center's Molecular Genetics Core Facility is gratefully acknowledged. This is ANDEEP publication number 10. This work was supported by grants from the Swiss National Science Foundation (31-59145.99), the Research Council of Norway (141050/730), and the National Science Foundation (OPP-9725830 and OPP-0003639).
Abbreviations: SSU, small subunit; NJ, neighbor joining; ML, maximum likelihood; Ma, mega-annum.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AF381179–AF381183, AJ307741–AJ307772, AJ311212–AJ311219, AJ312436, AJ315955, AJ317881, AJ317980, AJ317983–AJ317989, AJ318011–AJ318227, AJ504681–AJ504690, AJ514835–AJ514865, X86093, X86095, Z69610, and Z69613).
References
- 1.Ayala, F. J. & Rzhetsky, A. (1998) Proc. Natl. Acad. Sci. USA 95, 606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterson, K. J. & Davidson, E. H. (2000) Proc. Natl. Acad. Sci. USA 97, 4430–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heckman, D. S., Geiser, D. M., Eidell, B. R., Stauffer, R. L., Kardos, N. L. & Hedges, S. B. (2001) Science 293, 1129–1133. [DOI] [PubMed] [Google Scholar]
- 4.Sen Gupta, B. (1999) in Modern Foraminifera, ed. Sen Gupta, B. (Kluwer, Dordrecht, The Netherlands), pp. 7–36.
- 5.Culver, S. J. (1993) in Fossil Prokaryotes and Protists, ed. Lipps, J. (Blackwell Scientific, Boston), pp. 203–248.
- 6.Ross, C. & Ross, J. (1991) BioSystems 25, 39–51.1854913 [Google Scholar]
- 7.Culver, S. J. (1991) Science 254, 689–691. [DOI] [PubMed] [Google Scholar]
- 8.Culver, S. J. (1994) J. Foraminiferal Res. 24, 191–202. [Google Scholar]
- 9.Hansen, H. (1979) Lethaia 12, 173–182. [Google Scholar]
- 10.Tappan, H. & Loeblich, A. R., Jr. (1988) J. Paleontol. 62, 695–714. [Google Scholar]
- 11.Cherchi, A. & Schroeder, R. (1985) Bolletino Soc. Paleont. Italiana 23, 149–160. [Google Scholar]
- 12.McIlroy, D., Green, O. & Brasier, M. (2001) Lethaia 34, 13–29. [Google Scholar]
- 13.Pawlowski, J., Bolivar, I., Fahrni, J., De Vargas, C. & Bowser, S. S. (1999) Nature 399, 27. [DOI] [PubMed] [Google Scholar]
- 14.Pawlowski, J., Bolivar, I., Fahrni, J. F., De Vargas, C. & Bowser, S. S. (1999) J. Eukaryot. Microbiol. 46, 612–617. [DOI] [PubMed] [Google Scholar]
- 15.Pawlowski, J., Fahrni, J., Brykczynska, U., Habura, A. & Bowser, S. S. (2002) Polar Biol. 25, 96–105. [Google Scholar]
- 16.Galtier, N., Gouy, M. & Gautier, C. (1996) Comput. Appl. Biosci. 12, 543–548. [DOI] [PubMed] [Google Scholar]
- 17.Kimura, M. (1980) J. Mol. Evol. 16, 111–120. [DOI] [PubMed] [Google Scholar]
- 18.Felsenstein, J. (1984) Evolution 38, 16–24. [DOI] [PubMed] [Google Scholar]
- 19.Felsenstein, J. (1985) Evolution 39, 783–791. [DOI] [PubMed] [Google Scholar]
- 20.Swofford, D. (1998) paup*, Phylogenetic Analyses Using Parsimony (*and Other Methods) (Sinauer, Sunderland, MA).
- 21.Lanave, C., Preparata, G., Saccone, C. & Serio, G. (1984) J. Mol. Evol. 20, 86–93. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez, F., Oliver, J. L., Marin, A. & Medina, J. R. (1990) J. Theor. Biol. 142, 485–501. [DOI] [PubMed] [Google Scholar]
- 23.Posada, D. & Crandall, K. A. (1998) Bioinformatics 14, 817–818. [DOI] [PubMed] [Google Scholar]
- 24.Robinson-Rechavi, M. & Huchon, D. (2000) Bioinformatics 16, 296–297. [DOI] [PubMed] [Google Scholar]
- 25.Felsenstein J. (1988) Annu. Rev. Genet. 22, 521–565. [DOI] [PubMed] [Google Scholar]
- 26.Pawlowski, J., Holzmann, M., Berney, C., Fahrni, J., Cedhagen, T. & Bowser, S. S. (2002) J. Foraminiferal Res. 32, 334–343. [Google Scholar]
- 27.DeLaca, T. E., Bernhard, J., Reilly, A. & Bowser, S. S. (2002) J. Foraminiferal Res. 32, 177–187. [Google Scholar]
- 28.Keeling, P. J. (2001) Mol. Biol. Evol. 18, 1551–1557. [DOI] [PubMed] [Google Scholar]
- 29.Archibald, J. M., Longet, D., Pawlowski, J. & Keeling, P. J. (2003) Mol. Biol. Evol. 20, 62–66. [DOI] [PubMed] [Google Scholar]
- 30.Berney, C. & Pawlowski, J. (2003) J. Mol. Evol. 57, 1–8.12962301 [Google Scholar]
- 31.Travis, J. L. & Bowser, S. S. (1991) in Biology of Foraminifera, eds. Lee, J. J. & Anderson, O. R. (Academic, London), pp. 91–155.
- 32.Lipps, J. H. (1983) in Biotic Interactions in Recent and Fossil Benthic Communities, ed. McCall, P. (Plenum, New York), pp. 331–376.
- 33.Pawlowski, J., Bolivar, I., Fahrni, J. F., de Vargas, C., Gouy, M. & Zaninetti, L. (1997) Mol. Biol. Evol. 14, 498–505. [DOI] [PubMed] [Google Scholar]
- 34.Gutschick, R. (1986) J. Paleontol. 60, 233–248. [Google Scholar]
- 35.Butterfield, N. (2000) Paleobiology 26, 386–404. [Google Scholar]
- 36.Porter, S. & Knoll, A. (2000) Paleobiology 26, 360–385. [Google Scholar]
- 37.Knoll, A. (1994) Proc. Natl. Acad. Sci. USA 91, 6743–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schultze, M. (1854) Ueber den Organismus der Polythalamien (Foraminiferen), nebst Bemerkungen über die Rhizopoden im Allgemeinen (Wilhelm Engelmann, Leipzig).
- 39.Mikhalevich, V. & Debenay, J. (2001) J. Micropaleontol. 20, 13–28. [Google Scholar]
- 40.Hottinger, L. (2000) Micropaleontology 46, 57–86. [Google Scholar]
- 41.Rasmussen, B., Bengtson, S., Fletcher, I. R. & McNaughton, N. J. (2002) Science 296, 1112–1115. [DOI] [PubMed] [Google Scholar]
- 42.Gaucher, C. & Sprechmann, P. (1999) Beringeria 23, 55–91. [Google Scholar]
- 43.DeLaca, T. E. (1982) Am. Zoologist 22, 683–690. [Google Scholar]
- 44.Goldstein, S. (1999) in Modern Foraminifera, ed. Sen Gupta, B. (Kluwer, Dordrecht, The Netherlands), pp. 37–55.
- 45.Bowser, S. S., Alexander, S. P., Stockton, W. & DeLaca, T. E. (1992) J. Protozool. 39, 724–732. [Google Scholar]
- 46.Gooday, A. J., Bowser, S. S. & Bernhard, J. M. (1996) Prog. Oceanog. 37, 117–166. [Google Scholar]
- 47.Dayton, P. & Oliver, J. (1977) Science 197, 55–58. [DOI] [PubMed] [Google Scholar]
- 48.Lee, J. J. & Anderson, O. R. (1991) in Biology of Foraminifera, eds. Lee, J. J. & Anderson, O. R. (Academic, London), pp. 157–222.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.