Abstract
The role of the hepatitis C virus (HCV) p7 protein in the virus life cycle is not known. Previous in vitro data indicated that this 63-aa polypeptide is located in the endoplasmic reticulum and has two transmembrane domains (TMDs) connected by a cytoplasmic loop; the amino- and carboxyl-terminal tails are oriented toward the endoplasmic reticulum lumen. Furthermore, recent in vitro studies suggested that HCV p7 could function as a virus-encoded ion channel. It might therefore be a relevant target for future drug development. We studied the role of HCV p7 in vivo. Because HCV does not replicate efficiently in cell culture, we mutagenized p7 of an infectious genotype 1a cDNA clone and tested RNA transcripts of each mutant for infectivity in chimpanzees by intrahepatic transfection. Appropriate processing of mutant polypeptides was confirmed by studies in transfected mammalian cells. Mutants with deletions of all or part of p7 and a mutant with substitutions of two conserved residues in the cytoplasmic loop were not viable. Thus, p7 is essential for infectivity of HCV. A chimera in which the p7 of the 1a clone was replaced with p7 from an infectious genotype 2a clone also was not viable. This finding suggests a genotype-specific interaction between p7 and other genomic regions. To define which portions of p7 played the most significant role for this interaction, we tested three chimeras with the 1a backbone in which only specific domains of p7 had the 2a sequence. A p7 chimera with 2a tails and TMDs and the 1a cytoplasmic loop was not viable. A mutant with 2a tails and cytoplasmic loop and 1a TMDs also was not viable. However, a p7 chimera with 2a TMDs and cytoplasmic loop and 1a tails was viable. The transfected chimpanzee became viremic at week 2, and recovered viruses had the chimeric sequence. These data indicate that the amino- and/or carboxyl-terminal intraluminal tails of p7 contain sequences with genotype-specific function.
Hepatitis C virus (HCV), a member of the Flaviviridae family, is a major cause of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma worldwide. HCV is an enveloped virus with a positive single-stranded RNA genome that encodes a single polyprotein precursor of ≈3,000 aa that is cleaved into 10 proteins by host or viral proteases (1, 2). These include three structural proteins [one core (C) and two envelope (E1 and E2) glycoproteins], as well as six nonstructural proteins (NS2–NS5B) involved with polyprotein processing and RNA replication. However, it is not known whether the 10th protein, p7, is a structural or nonstructural protein. Studies of HCV subgenomic replicons showed that p7 is not critical for RNA replication (3), but its actual role in the virus life cycle has not been determined. The corresponding protein of bovine viral diarrhea virus (BVDV), another member of the Flaviviridae family, was critical for cell culture infectivity (4). Transfection with RNA transcripts from an infectious BVDV cDNA clone with in-frame deletions or point mutations of p7 could not produce infectious virus, but the infectivity could be restored by providing p7 in trans.
The p7 gene of HCV is located between the E2 and NS2 genes; it encodes a hydrophobic protein consisting of 63 aa. The cleavage of p7 is mediated by the endoplasmic reticulum (ER) signal peptidases of the host cell (5–7). Most cleavages in the polyprotein precursor occur during or immediately after translation; however, cleavages at the E2/p7 and p7/NS2 sites are delayed, resulting in the production of an E2–p7–NS2 precursor protein (8). Furthermore, incomplete cleavage at the E2/p7 site results in a mixture of an E2–p7 protein as well as the individual proteins (5, 7). It is not known whether E2–p7 performs a specific function. However, the E2–p7 protein of BVDV was not essential for cell culture infectivity, because RNA transcripts from a bicistronic construct in which E2 and p7 were expressed separately could produce infectious virus (4).
The topology of the HCV p7 polypeptide has been examined in vitro (9). Studies of p7 expressed in mammalian cells indicated that p7 is a membrane-spanning protein located in the ER. Accordingly, p7 has two transmembrane domains (TMDs) connected by a cytoplasmic loop; the amino- and carboxyl-terminal tails are oriented toward the ER lumen. It also has been demonstrated that the carboxyl-terminal TMD of p7 can function as a signal sequence that most likely promotes the translocation of NS2 into the ER lumen for appropriate cleavage by host signal peptidases. A recent in vitro study (10), however, found that NS2 could become membrane-associated in the absence of p7.
A recent study suggested that HCV p7 has characteristics similar to those of a group of proteins called viroporins (11). These proteins form ion channels that might be of importance for virus assembly and/or release. Griffin et al. (11) demonstrated that recombinant HCV p7 proteins could be cross-linked as hexamers in HepG2 cells and that Escherichia coli-expressed p7 also formed hexamers in vitro. Furthermore, it was demonstrated that recombinant HCV p7 could form ion channels in artificial lipid membranes, suggesting that this protein could be responsible for ion transport from the ER into the cytoplasm of HCV-infected host cells. Interestingly, the ion transport observed in lipid membranes could be blocked by a known ion channel inhibitor, amantadine, which might have a beneficial effect in HCV treatment (11, 12). Furthermore, inhibition of the p7 ion channel activity was demonstrated with various long-alkyl-chain aminosugar derivatives (13), suggesting that HCV p7 could be a highly relevant target for antiviral drug development. Without a cell culture system to generate virus particles, however, it will be difficult to study the role of the p7 ion channels further, and studies of other viral proteins with ion channel activity (e.g., the NB protein of influenza B) have indicated that such proteins might not necessarily be critical for virus propagation (14). Thus, it is important to determine whether p7 is critical for infectivity of HCV.
In the absence of a reliable cell culture system or of a small animal model to propagate HCV, we and others have previously performed studies in chimpanzees to determine the importance of genetic elements of HCV (15–20). In these studies, infectious clones of HCV were mutagenized and infectivity of mutants was tested by transcribing genomic RNA in vitro and inoculating it directly into the liver of chimpanzees. In the present study, we used this strategy to demonstrate that p7 is critical for infectivity of HCV. Furthermore, because p7 exhibits significant genetic heterogeneity among isolates belonging to different genotypes, we examined whether the p7 protein contains sequences with a genotype-specific function.
Materials and Methods
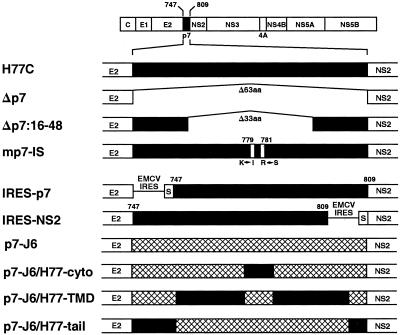
Construction of HCV Expression Vectors. Processing of E2–p7–NS2 mutants derived from the infectious clone of genotype 1a (pCVH77C) (21) was studied with subgenomic expression vectors generated by standard PCR and cloning procedures. We used pcDNA3.1(+) (Invitrogen) to generate 10 expression vectors with HCV inserts downstream from the cytomegalovirus (CMV) immediate-early enhancer/promoter and T7 RNA polymerase-binding site. The correctness of all vectors was verified by restriction digest analysis and sequencing. The wild-type clone and nine mutants (Fig. 1) were constructed as described in the Supporting Text, by using the primers listed in Table 2, both of which are published as supporting information on the PNAS web site, www.pnas.org. The pc.wt vector encoded the carboxyl-terminal 20 aa of E1 (signal sequence) and the entire E2, p7, and NS2 proteins of HCV (amino acids 364-1026). The pc.Δp7 plasmid lacked p7 (amino acids 747–809). The pc.Δp7:16-48 clone lacked the central 33 aa (amino acids 763–795) of p7. The construct pc.mp7-IS had two amino acid substitutions (K779I and R781S) in the cytoplasmic loop of p7. The bicistronic construct pc.IRES-p7 contained a stop codon after the HCV E2, followed by the internal ribosome entry site element (IRES) of encephalomyocarditis virus, a signal peptide of the mouse Ig κ chain, HCV p7, and HCV NS2. In the second bicistronic construct, pc.IRES-NS2, the same IRES and signal peptide sequence were inserted between p7 and NS2. In the chimera pc.p7-J6, p7 from the 1a clone was replaced with p7 from an infectious clone of genotype 2a (18). In the chimera pc.p7-J6/ H77-tail, a subdomain of the 1a p7 (nucleotides 37–173 of p7) was replaced with 2a sequence. This plasmid had a single silent mutation [nucleotide 174 (T to A)]. In pc.p7-J6/H77-cyto, two domains of the 1a p7 (nucleotides 1–93 and 118–189 of p7) were replaced with 2a sequence. Finally, in the pc.p7-J6/H77-TMD chimera, three domains of the 1a p7 (nucleotides 1–36, 94–117, and 175–189 of p7) were replaced with 2a sequences.
Fig. 1.
Schematic representation of the mutant p7 sequences. The location of p7, encoding amino acids 747–809, in H77C is indicated (black box) at the top. White boxes in mp7-IS indicate amino acids that were mutated. Hatched boxes indicate the genotype 2a p7 sequences substituted from pJ6CF. The p7-J6/H77-tail chimera was the only viable construct among the mutants tested. EMCV IRES, IRES of encephalomyocarditis virus. S, signal peptide of the mouse Ig κ chain.
Processing of HCV Mutant Proteins in Mammalian Cells. Huh-7 cells and 293T cells were cultured in Dulbecco's modified Eagle's medium supplemented with 2 mM l-glutamine, 1% penicillin/streptomycin, and 10% FBS. Cells were transfected at 60–80% confluence in six-well plates with each expression vector or with pcDNA3.1(+) (without HCV sequences) by using Superfect Transfection Reagent (Qiagen, Valencia, CA). After ≈48 h cells were harvested, washed with PBS twice, resuspended in 500 μl of PBS, and then used for further analysis or stored at –80°C.
Endoglycosidase H (endo H) digestion was performed according to the manufacturer's instructions (New England Biolabs). Briefly, 5 μl of cell suspension was added to 2 μl of 10× denaturing buffer and 13 μl of water, heated at 100°C for 10 min, and then chilled on ice. Samples were mixed with 1 μl of endo H and 2.3 μl of 10× G5 buffer (50 mM sodium citrate, pH 5.5, at 25°C) and incubated at 37°C for 80 min. The protein mixtures then were analyzed by SDS/PAGE.
For Western blotting, cell suspensions were lysed and denatured in 1× LDS buffer [10% glycerol/141 mM Tris base/106 mM Tris·HCl/2% lipoprotein-deficient serum/0.51 mM EDTA/0.22 mM Serva Blue G250/0.175 mM phenol blue (pH 8.5); Invitrogen] at 70°C for 10 min. Denatured sample was submitted to SDS/PAGE in NuPAGE 4–12% Bis-Tris Gels (Invitrogen). Proteins were transferred to nitrocellulose membranes. Membranes were incubated with anti-hypervariable region 1 (HVR1) rabbit hyperimmune serum (LMF86) (22) or anti-NS2 rabbit hyperimmune serum (WU107, kindly provided by Charles M. Rice, The Rockefeller University, New York) (23) at a 1:2,000 dilution at room temperature for 1 h. By following standard washing procedures, membranes were incubated with a goat anti-rabbit Ig conjugated to horseradish peroxidase (Pierce) at a 1:5,000 dilution at room temperature for 1 h. After washing, the membranes were incubated with substrate and exposed to films.
Construction of Full-Length H77C Clones with Mutated p7. The mutated p7 of the various expression vectors described above was substituted for the corresponding sequence of pCV-H77C (21) by using AscI and AfeI sites. Each mutant DNA was transfected into DH5α cells, and transformed colonies were cultured in LB liquid containing ampicillin (21). After small-scale preparation, a plasmid was retransformed to select a single clone, and large-scale preparation of plasmid DNA was performed with a Qiagen EndoFree Maxi Kit (21). For each mutant, the complete sequence of the final DNA preparation was confirmed.
Analysis of H77C Genomes with Mutated p7 in Chimpanzees. In each of two reactions, 10 μg of plasmid DNA, linearized by XbaI (Promega) digestion, was transcribed in a 100-μl reaction volume with T7 RNA polymerase (Promega) at 37°C for 2 h (21), and then 5 μl of the final reaction mixture was analyzed by agarose gel electrophoresis. Each transcription mixture was diluted with 400 μl of PBS and then immediately frozen on dry ice and stored at –80°C. Within 24 h, transcription mixtures were injected into a chimpanzee by percutaneous intrahepatic injection guided by ultrasound (16). If the chimpanzee did not become infected, the transfection was repeated once. The first six mutants were tested individually, but the three mutants in which only subdomains of the 1a p7 were replaced with 2a sequences were tested simultaneously. The animals were maintained in an approved facility under conditions that met or exceeded all requirements for animal use.
Liver biopsies and serum samples were collected twice weekly from the chimpanzees. Liver biopsies were examined for necroinflammatory changes as described (19). Serum samples were tested for HCV antibodies with second-generation ELISA (Abbott) and for alanine aminotransferase (ALT) levels (Anilytics, Gaithersburg, MD). Serum HCV RNA was monitored in RT-nested PCR specific for the 5′ UTR (24). The limit of detection of this assay is ≈10 genome equivalents per ml. The HCV RNA titer was quantified by the HCV Monitor Amplification Kit Version 2.0 (Roche Diagnostics). The lower detection limit of this test is 600 units/ml (≈500 genome equivalents per ml).
When chimpanzee 1625 became infected after transfection with the mixture of three 1a/2a chimeras, a genome region (nucleotides 2443–2779 and amino acids 702–812), including p7, of recovered viruses was amplified in RT-nested PCR with H77-specific primers [I PCR, 2404F_1a (GCCTCATCCACCTCCACCAGA) and 2467R_1a (AGAACAACGCCGCCACACG); II PCR, 2420F_1a (CCAGAACATTGTGGACGTGCAGT) and 2457R_1a (CGCCACACGACGCGGCCACC)]. The sensitivity of this assay for H77 was equivalent to that of the 5′ UTR RT-nested PCR. Amplicons were sequenced directly or cloned by the TOPO TA Cloning Kit for Sequencing (Invitrogen) and sequenced. The entire ORF of virus recovered from the serum of chimpanzee 1625 at selected weeks also was analyzed by amplifying 19 overlapping subgenomic regions of HCV by RT-nested PCR with H77-specific primers, and amplicons were sequenced directly to obtain consensus sequences.
Results
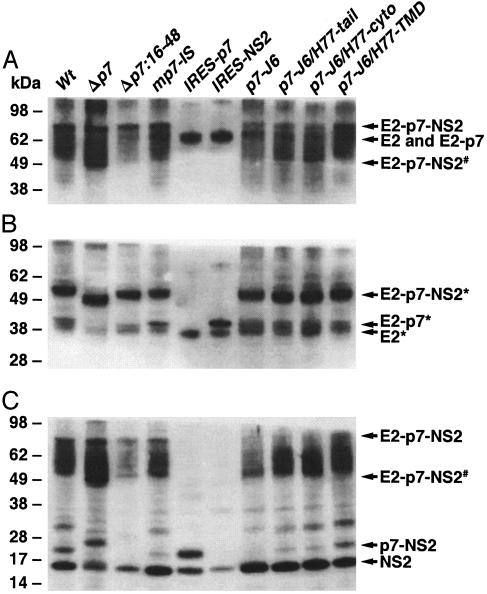
p7 Mutants Are Processed Appropriately in Mammalian Cells. The purpose of our study was to test the viability of HCV cDNA with mutagenized p7. Thus it was important to demonstrate that introduced changes did not significantly affect the polyprotein processing. Processing at the E2/p7 and p7/NS2 sites is mediated by host signal peptidase in the ER. It was previously determined that cleavage between E2 and p7 was incomplete, so it follows that E2, E2–p7, and p7 are produced from the E2–p7–NS2 precursor. For all p7 mutants/chimeras generated, we tested whether the in vitro-expressed precursor E2–p7–NS2 was processed as predicted in mammalian cells. Expression vectors containing the E2 through NS2 genes (Fig. 1) were transiently transfected into Huh-7 or 293T cells, and expressed proteins were subsequently analyzed by SDS/PAGE and Western blotting. Only the data obtained in 293T cells are shown, but similar results were obtained in Huh-7 cells. Using anti-hypervariable region 1 (HVR1) hyperimmune serum, we confirmed that all constructs produced glycosylated precursor (Fig. 2A). However, because the sizes of glycosylated E2 and E2–p7 proteins were similar, we could not readily distinguish between these proteins. Therefore, we deglycosylated the expressed proteins and repeated the Western blotting with anti-HVR1 (Fig. 2B). These experiments showed that the cleavage between E2 and p7 was incomplete. In the analysis of pc.Δp7 (deletion of the entire p7 protein) and pc.IRES-p7 (bicistronic construct expressing E2 and p7 individually), only the E2 protein was detected, whereas in the analysis of the eight other constructs, including the wild-type, both the E2 and E2–p7 proteins were detected. For pc.Δp7:16-48 (deletion of the middle portion of the p7 protein), it was clear that the E2–p7 protein was smaller than that observed for the other constructs (Fig. 2B). Using anti-NS2 hyperimmune serum, we confirmed that the cleavage between p7 and NS2, or E2 and NS2, occurred in all constructs (Fig. 2C). It is noteworthy that the amounts of E2 and NS2 proteins in pc.Δp7 were low compared with those seen with the other constructs, suggesting that deletion of the entire p7 protein did decrease the efficiency of cleavage. Overall, each mutant had the expected processing products after transient expression in mammalian cells, suggesting that the polyprotein encoded by the full-length cDNA with p7 alterations should be processed appropriately in vivo.
Fig. 2.
Analysis of expressed HCV proteins by Western blotting. Approximately 48 h after transfection of 293T cells with pc.wt and mutant expression vectors, cells were lysed and submitted to SDS/PAGE. The positions of HCV proteins are indicated on the right. Sizes (in kDa) of marker protein are indicated on the left. (A) The primary antibody was a rabbit hyperimmune serum against E2 hypervariable region 1 (HVR1) (LMF86). (B) After endoglycosidase H (endo H) treatment, samples were submitted to SDS/PAGE. The primary antibody was LMF86. (C) The primary antibody was a rabbit hyperimmune serum against NS2 (WU107). #, Unglycosylated precursor protein; *, deglycosylated protein.
The HCV p7 Protein Is Critical for Infectivity in Chimpanzees. We introduced the p7 mutations into an infectious clone of genotype 1a, pCV-H77C (21) (Fig. 1), and tested infectivity of RNA transcripts by consecutive intrahepatic transfections of a chimpanzee. Mutants were considered not viable if viremia, as measured by detection of viral RNA in the serum by RT-nested PCR, was not detected after two independent transfections. A mutant in which the entire p7 protein (amino acids 747–809) was deleted [H77C(Δp7)] was not viable, as was a mutant in which only the middle part of p7 (amino acids 763–795), including sequences encoding portions of the two TMDs and the entire cytoplasmic loop, was deleted [H77C(Δp7:16-48)]. This second construct permitted more efficient processing in vitro of the E2 and NS2 proteins compared with the construct in which the entire p7 protein was deleted (see above).
The cytoplasmic loop of p7 contains positively charged amino acid residues at positions 779 and 781. At these amino acid positions, all isolates of HCV, irrespective of genotype, have either lysine or arginine. We found that the mutant H77C(mp7-IS), which encoded noncharged amino acids at these positions (K779I and R781S), was not viable in the chimpanzee. Overall, our data indicate that the p7 protein of HCV is critical for infectivity in vivo.
Attempt to Determine Whether the Unprocessed E2-p7 Protein Is Critical for Infectivity of HCV. In vitro studies indicated that the p7 protein also exists as unprocessed E2–p7. In an attempt to determine whether this protein had a function in infectivity of HCV, we tested H77C(IRES-p7), a bicistronic construct that encoded the individual E2 and p7 proteins but not E2–p7. Two independent intrahepatic transfections of a chimpanzee with RNA transcripts from this mutant did not result in infection. These data could suggest that E2–p7 is critical for HCV infectivity. However, it is also possible that this clone was not viable merely because of the heterologous sequences inserted into the HCV genome. Therefore, in an attempt to rule out this explanation, we tested another bicistronic construct, H77C(IRES-NS2), in which the exact heterologous sequence was introduced instead between the p7 and NS2 genes to produce a molecular clone of the same size as that of IRES-p7. The resulting construct could encode the E2, p7, and unprocessed E2–p7 proteins. Because this bicistronic construct was also not viable in the chimpanzee, we cannot reach a conclusion regarding the role of the unprocessed E2–p7 protein.
The p7 Protein of HCV Contains Genotype-Specific Sequences That Interact with Other Genomic Regions. To determine whether p7 contains important genotype-specific sequences, we tested the infectivity of a chimeric molecular clone, H77C(p7-J6), in which the p7 gene of the 1a clone was replaced with p7 from an infectious 2a clone (pJ6CF) (18). The 1a and 2a p7 sequences differ at 81 (42.9%) nucleotide positions and at 24 (38.1%) amino acid positions (Fig. 3). This intertypic 1a/2a chimeric sequence was not infectious in the chimpanzee, suggesting a genotype-specific interaction between p7 and other genome regions.
Fig. 3.
Alignment of amino acid sequences of p7 from intertypic 1a/2a constructs. The sequence of H77C is shown on top. The differences from H77C are indicated below. The sequence of viruses recovered from chimpanzee 1625, transfected with p7-J6/H77-cyto, p7-J6/H77-TMD, and p7-J6/H77-tail, is shown at the bottom. Four positions with universally conserved genotype 2-specific sequences are indicated.
To define which portions of p7 played the most significant role for this interaction, we tested three additional 1a chimeras in which only specific domains of p7 had the 2a sequence: a p7 with 2a tails and TMDs and the 1a cytoplasmic loop [H77C(p7-J6/H77-cyto)], a p7 with 2a tails and cytoplasmic loop and 1a TMDs [H77C(p7-J6/H77-TMD)], and a p7 with 2a TMDs and cytoplasmic loop and 1a tails [H77C(p7-J6/H77-tail)] (Figs. 1 and 3). The infectivity of RNA transcripts from the three chimeras was tested simultaneously in chimpanzee 1625 (Fig. 4). The chimpanzee became viremic at week 2 postinoculation, as measured by two different RT-nested PCR assays. The less sensitive Monitor test did not become positive until week 3.5. The animal became persistently infected, according to serum HCV RNA-positive tests, throughout the follow-up period of 40 weeks. The viremia titers remained at relatively low levels. The highest acute phase HCV RNA titer was observed at week 6.5 and was only 103.7 units/ml, compared with peak titers of ≈105.5 units/ml we observed previously in two animals infected with the wild-type 1a clone. Furthermore, the animal had late seroconversion. The second-generation ELISA did not become positive until week 35, whereas the animals infected with the wild-type 1a clone became positive during weeks 13 and 15, respectively. Liver enzyme values were only marginally elevated during the acute infection [peak alanine aminotransferase (ALT) level of 59 units/liter], but histological examination showed evidence of mild hepatitis during weeks 11–14. The two animals with wild-type 1a infection had peak ALT levels of 133 and 164 units/liter, respectively, and had evidence of mild to moderate hepatitis in liver biopsies. Thus, the HCV genotype 1a genome with chimeric intertypic 1a/2a p7 seemed to be attenuated in vivo.
Fig. 4.
Course of HCV infection in chimpanzee 1625. The animal became infected after transfection with RNA transcripts of a p7 chimera with 2a TMDs and cytoplasmic loop and 1a tails (p7-J6/H77-tail). Serum samples were tested for HCV RNA by RT-nested PCR with 5′ UTR primers and/or by the Roche Diagnostics Monitor test. Filled rectangle, positive by RT-nested PCR and/or by Monitor test; open rectangle, negative by RT-nested PCR in two independent assays; open circle, HCV Monitor titers. Samples found to be below the detection limit of 600 units/ml (dashed line) are shown as not detected (ND). All samples with a low titer (600–1,000 units/ml) in the Monitor test were confirmed positive in the RT-nested PCR assay. Seroconversion in the second-generation ELISA for anti-HCV is represented by a horizontal bar. Shaded area, serum alanine aminotransferase (ALT). For liver histology, necroinflammatory changes of liver biopsy samples were graded as 0 (normal), 1 (mild), 2 (mild to moderate), 3 (moderate to severe), or 4 (severe).
Sequence analysis of viruses recovered from chimpanzee 1625 demonstrated that only RNA transcripts from p7-J6/H77-tail were infectious (Fig. 3). A region encoding amino acids 702–812, including p7, was amplified from serum collected twice weekly during weeks 2–20 and was sequenced directly to obtain a consensus sequence, and clonal analysis was performed at selected weeks (Table 1). At week 2, the consensus sequence was identical with the p7-J6/H77-tail sequence (Fig. 3), and all 91 clones analyzed originated from this clone (Table 1). Also, only genomes that originated from this chimeric p7 sequence, among a total of 359 clones, were recovered from the animal at 14 additional time points (Table 1). Thus, a chimeric 1a molecular clone in which the TMDs and cytoplasmic loop of p7 were replaced with 2a sequences was viable in vivo. Our data indicate that the amino- and/or carboxyl-terminal tails of p7 contain sequences with genotype-specific function. Interestingly, the p7 tails of all published sequences of genotype 2 isolates contain three invariable amino acids (K750, H755, and Q806) that are not found in isolates representing other genotypes (Fig. 3).
Table 1. Evolution of 1a/2a intertypic p7 chimera in chimpanzee 1625.
| p7
|
NS2
|
NS3
|
NS4B
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 795 | 796 | 918 | 1202 | 1531 | 1745 | 1763 | 1906 | |||
| H77C | P | L | L | G | T | V | K | V | ||
| J6CF | S | F | L | D | T | D | K | V | ||
| p7-J6/H77-tail | S | F | L | G | T | V | K | V | ||
| Weeks p.i. | ||||||||||
| 2 | S | 90S/1P | F | 91F | ||||||
| 2.5 | S | 32S | L/f | 20L/12F | ||||||
| 3 | S | 19S | L/f | 15L/3F/1I | ||||||
| 3.5 | S | 16S | L/f | 9L/7F | ||||||
| 4 | S | 34S | L/f | 29L/5F | ||||||
| 4.5 | S | 23S/1P | L | 23L/1F | ||||||
| 5 | S | 19S | L | 19L | ||||||
| 5.5 | S | L | ||||||||
| 6 | S | L | ||||||||
| 6.5 | S | 34S/2P | L | 36L | L/i | G | T | V | K/r | V |
| 7 | S | L | ||||||||
| 7.5 | S | L | ||||||||
| 8 | S | 21S | L | 21L | ||||||
| 8.5 | S | L | ||||||||
| 9 | S | L | ||||||||
| 9.5 | S | 24S | L | 24L | ||||||
| 10 | S | L | ||||||||
| 10.5 | S | L | ||||||||
| 11 | S/p | 31S/16P | L | 47L | F | E | A | V | R/k | V |
| 11.5 | S/p | L | ||||||||
| 12 | S/p | 15S/3P | L | 18L | ||||||
| 12.5 | S/p | L | ||||||||
| 13 | S/p | L | ||||||||
| 13.5 | ||||||||||
| 14 | S/p | L | ||||||||
| 14.5 | S/p | 21S/3P | L | 24L | F | E | A | V/a | R/k | A |
| 15 | S/p | L | ||||||||
| 15.5 | S | L | ||||||||
| 16 | S | L | ||||||||
| 16.5 | S | L | ||||||||
| 17 | S/p | L | ||||||||
| 17.5 | S/p | L | ||||||||
| 18 | S/p | L | ||||||||
| 18.5 | S/p | 22S/2P | L | 24L | F | E | A/t | A/v | R/k | A |
| 19 | S/p | L | ||||||||
| 19.5 | S | L | ||||||||
| 20 | S | 21S | L | 21L | ||||||
The consensus sequence of the ORF of viruses recovered from serum at weeks 6.5, 11, 14.5, and 18.5 posttransfection was determined. Only amino acid changes are given. The corresponding amino acids and their position in the H77C (infectious 1a clone) polyprotein are shown in the first row, followed by the sequence of J6CF (infectious 2a clone) and the viable p7 chimera p7-J6/H77-tail in the second and third rows, respectively. The differences from the p7-J6/H77-tail plasmid sequence in sequences recovered from chimpanzee 1625 are indicated below: dominant sequences are shown in capital letters, and minor sequences are shown in lowercase letters. For sequential analysis of the p7 positions with heterogeneity, the consensus sequence of a subgenomic region (amino acids 702-812) was determined. Clonal analysis of p7 also was performed, and the results are indicated to the right of positions with changes observed in the consensus sequence. Note that at position 795 the consensus sequence did not change, but significant quasispecies were observed. p.i., Postinoculation.
The consensus sequence of the HCV ORF was determined at weeks 6.5, 11, 14.5, and 18.5 (Table 1), the four time points with the highest viral titers during the first 20 weeks. Only a single nucleotide change (T2729A), in p7, was detected at week 6.5 and thereafter. This change resulted in an amino acid change from the 2a to the 1a sequence in the second TMD of p7 (F796L). This mutation appeared at week 2.5 (Fig. 3 and Table 1). The original sequence was found as a minor species during weeks 2.5–4.5, but not thereafter. At week 5 and later time points, only the leucine at position 796 was detected. Interestingly, this is the only other invariable genotype 2-specific sequence in p7 (Fig. 3), and the observed change most likely compensated partially for the attenuation of the transfected chimera. The only other evidence of evolution in the chimeric p7 was significant quasispecies at position 2724 (T/c) which resulted in the 1a sequence being present as a minor species at amino acid 795 (S/p) during weeks 11–19. However, the dominant sequence at this position remained the 2a sequence during the first 20 weeks of follow-up (Table 1). Outside p7, five additional nucleotide changes (C2894G, C3093T, G3946A, A4932G, and A5629G), four of which resulted in amino acid changes (L918F, G1202E, T1531A, and K1763R), were detected at week 11 (Table 1). Two additional nonsilent changes (T6058C and T5575C) were found at week 14.5 (V1906A) and week 18.5 (V1745A), respectively. The 1a and 2a clones have an identical sequence at four of the six amino acid positions where changes occurred outside p7 (Table 1). At the two remaining positions, the changes did not result in changes from the 1a backbone to the 2a sequence.
Discussion
We mutagenized p7 of an infectious genotype 1a cDNA clone and tested mutant RNA for infectivity in chimpanzees. Our data indicate that the p7 protein of HCV is essential for infectivity and contains important genotype-specific sequences. Mutants with deletions of all or part of p7 and a mutant with substitutions of two conserved residues in the cytoplasmic loop were not viable. A chimera in which the p7 of the 1a clone was replaced with p7 from an infectious 2a clone also was not viable. However, a mutant in which only the TMDs and cytoplasmic loop, but not the tails, of p7 of the 1a clone were replaced with the 2a sequences was viable.
It was previously reported that the corresponding p7 protein of BVDV, another member of the Flaviviridae family, was not required for RNA replication but was essential for the generation of infectious virus in cell culture (4). A mutant BVDV cDNA with an in-frame deletion in p7 and mutants with point mutations of charged amino acids in a domain equivalent to the cytoplasmic loop of the HCV p7 were able to replicate in cell culture but did not spread. Providing the p7 protein in trans restored the infectivity of the deletion mutant, suggesting that the BVDV p7 does not contain important cis-acting elements. The HCV p7 also is apparently not important for RNA replication, because replicons without p7 replicate efficiently in Huh-7 cells (3, 25). Similar to the case with BVDV, we found that deletion of p7 or mutation of just two charged residues in the HCV p7 protein (K779 and R781), located in the cytoplasmic loop, rendered an infectious clone noninfectious. We believe that it is the p7 protein, and not the primary sequence, that is critical for infectivity, because the primary sequence encoding amino acids 779 and 781, the only two positions mutated in mp7-IS, exhibits significant heterogeneity at all six nucleotide positions among different isolates of HCV (24). Supporting this notion is the fact that the p7 sequence of HCV apparently does not contain important RNA secondary structures (26). The p7 proteins of HCV and BVDV most likely are required for virus assembly and/or release, as has been demonstrated for other viral proteins with similar properties (27).
We were interested in determining whether the unprocessed E2–p7 of HCV plays a critical role for the virus. It was reported that a BVDV bicistronic cDNA clone, which produced the E2 and p7 proteins separately, was infectious in cell culture (4), suggesting that E2–p7 was not required. We tested the infectivity of a similar bicistronic construct of HCV in which exactly the same heterologous sequence that was used for BVDV was inserted between E2 and p7 (IRES-p7, Fig. 1). However, this clone was not infectious, which could suggest that the E2–p7 protein of HCV is required for infectivity in vivo. However, because a construct that did express E2–p7 and contained an insertion of the same IRES sequence between p7 and NS2 also was not infectious, it is also possible that the increased genome length affected RNA packaging. The length of the bicistronic RNA transcripts was ≈10.2 kb, and the HCV genome of published isolates has a length of 9.6–9.7 kb. In contrast, the length of the BVDV genome is between 12.3 and 13.2 kb, and an isolate with a 15.5-kb genome has been described (GenBank ID code U86599). Thus, BVDV might have less stringent RNA packaging requirements than HCV. Alternatively, the heterologous sequence could have affected some important secondary structures of the HCV genome in both bicistronic constructs tested.
Recent studies performed in vitro with expressed protein in artificial lipid membranes suggest that the HCV p7 could perhaps function as an ion channel (11, 13). As such, p7 could be an additional target for drug development against HCV. However, proteins that can function as a virus-encoded ion channel in membranes do not necessarily have a critical function, as was recently reported for the M2 protein of the influenza A virus and the NB protein of the influenza B virus, two well characterized proteins with ion channel activity. It was found that the ion channel activity of the M2 and NB proteins was not essential for replication in cell culture (28, 29). Furthermore, a mutant of influenza A with defective M2 channel activity could replicate also in vivo, although the M2 protein was critical for infectivity (29). In contrast, the NB protein of the influenza B virus was found not to be essential for replication of the influenza B virus in cell culture or in vivo (14). Our data clearly demonstrated that p7 has an essential function for HCV. However, future studies are required to determine whether the ion channel activity of p7 is in fact a critical function and/or whether the p7 protein has additional essential functions as has been reported for other viral proteins with ion channel activity (30).
An important finding of our study was that p7 of HCV contains genotype-specific sequences that interact with sequences of other genome regions. The 1a/2a chimera containing the entire 2a p7 protein was nonviable, whereas a chimera containing only the p7 TMDs and cytoplasmic loop of 2a was viable. Both mutants were processed as expected at the E2/p7 and p7/NS2 sites. Therefore, the essential interaction was predicted to be between sequences of the luminal tails of p7 and RNA/protein sequences of other genomic regions. The two chimeras differ only at three amino acid positions (Fig. 3), and it is intriguing that at these three positions all genotype 2 isolates published have the same sequence (K750, H755, and Q806), whereas isolates of the other five major genotypes all have a different sequence at these sites. There is only one other such genotype 2-specific sequence in p7 (F796), located in the second TMD, and at this position the virus recovered from the chimpanzee had changed to the 1a sequence at week 2.5 (Fig. 3). We predict that introduction of one or more of these genotype 2-specific amino acids into the 1a backbone would render this clone nonviable, and it could be determined in future studies which of these positions are critical for interaction.
Recently, a naturally occurring intertypic chimera of HCV was identified (31). In this chimera, the 5′ end of the genome, including the sequence encoding the first 139 aa of NS2, was genotype 2k, whereas the remainder of the genome was genotype 1b. This observation suggests that the interaction of p7 could be with the 5′ UTR, C, E1, E2, and/or NS2 sequences. In vitro studies of protein–protein interactions could be a first step in identifying the nature of this interaction.
The relatively low viremia titers and late seroconversion suggested that the viable 1a/2a chimera of HCV was attenuated in vivo, as we have observed previously for a hypervariable region 1 (HVR1) deletion mutant (20). Appearance of viremia was delayed, and efficient replication was observed only after a mutation occurred in the second TMD of p7. However, even after this putative adaptive mutation appeared at week 2.5, the viral titers remained at low levels, suggesting that the chimeric p7 was not fully functional. Five additional mutations occurred outside p7, in NS2, NS3, and NS4B, during weeks 6.5–11 (Table 1). The mutation at amino acid 1202 most likely also provides a replicative advantage in chimpanzees, because this mutation appears early in the majority of chimpanzees transfected with the wild-type 1a clone (J.B., unpublished data). It is noteworthy that at the four other positions with changes, the 1a and 2a clones used to generate the p7 chimera have identical sequences. Although they could have represented changes that compensated for decreased replicative fitness of the chimera, they most likely represented selection after host cellular immune responses or random coselected mutations.
This study, which analyzed the role of the p7 polypeptide of HCV in vivo, demonstrated that the p7 protein of HCV plays an essential role in the virus life cycle and that this protein interacts with RNA/protein sequences in other genome regions. Current therapy of patients with hepatitis C, combination treatment with IFN and ribavirin, is successful in <50% of cases, and there is an urgent need for new treatment strategies against this frequent cause of chronic liver disease. Taken together with the recent evidence that recombinant p7 could function as an ion channel, our data on the in vivo relevance of this protein make the case that p7 should be an additional target for future drug development against HCV.
Supplementary Material
Acknowledgments
We thank Dr. Charles M. Rice for providing the WU107 rabbit hyperimmune serum and R. E. Engle for performing the Roche Diagnostics Monitor test. Analyses of HCV sequences were performed with the aid of the Hepatitis Virus Database (http://s2as02.genes.nig.ac.jp).
Abbreviations: HCV, hepatitis C virus; BVDV, bovine viral diarrhea virus; ER, endoplasmic reticulum; TMD, transmembrane domain; IRES, internal ribosome entry site element.
References
- 1.Major, M. E. & Feinstone, S. M. (1997) Hepatology 25, 1527–1538. [DOI] [PubMed] [Google Scholar]
- 2.Reed, K. E. & Rice, C. M. (2000) Curr. Top. Microbiol. Immunol. 242, 55–84. [DOI] [PubMed] [Google Scholar]
- 3.Lohmann, V., Korner, F., Koch, J., Herian, U., Theilmann, L. & Bartenschlager, R. (1999) Science 285, 110–113. [DOI] [PubMed] [Google Scholar]
- 4.Harada, T., Tautz, N. & Thiel, H. J. (2000) J. Virol. 74, 9498–9506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizushima, H., Hijikata, M., Asabe, S., Hirota, M., Kimura, K. & Shimotohno, K. (1994) J. Virol. 68, 6215–6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizushima, H., Hijikata, M., Tanji, Y., Kimura, K. & Shimotohno, K. (1994) J. Virol. 68, 2731–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin, C., Lindenbach, B. D., Pragai, B. M., McCourt, D. W. & Rice, C. M. (1994) J. Virol. 68, 5063–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubuisson, J., Hsu, H. H., Cheung, R. C., Greenberg, H. B., Russell, D. G. & Rice, C. M. (1994) J. Virol. 68, 6147–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrere-Kremer, S., Montpellier-Pala, C., Cocquerel, L., Wychowski, C., Penin, F. & Dubuisson, J. (2002) J. Virol. 76, 3720–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaga, A. K. & Ou, J. H. (2002) J. Biol. Chem. 277, 33228–33234. [DOI] [PubMed] [Google Scholar]
- 11.Griffin, S. D., Beales, L. P., Clarke, D. S., Worsfold, O., Evans, S. D., Jaeger, J., Harris, M. P. & Rowlands, D. J. (2003) FEBS Lett. 535, 34–38. [DOI] [PubMed] [Google Scholar]
- 12.Berg, T., Kronenberger, B., Hinrichsen, H., Gerlach, T., Buggisch, P., Herrmann, E., Spengler, U., Goeser, T., Nasser, S., Wursthorn, K., et al. (2003) Hepatology 37, 1359–1367. [DOI] [PubMed] [Google Scholar]
- 13.Pavlovic, D., Neville, D. C., Argaud, O., Blumberg, B., Dwek, R. A., Fischer, W. B. & Zitzmann, N. (2003) Proc. Natl. Acad. Sci. USA 100, 6104–6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatta, M. & Kawaoka, Y. (2003) J. Virol. 77, 6050–6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolykhalov, A. A., Mihalik, K., Feinstone, S. M. & Rice, C. M. (2000) J. Virol. 74, 2046–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanagi, M., St. Claire, M., Shapiro, M., Emerson, S. U., Purcell, R. H. & Bukh, J. (1998) Virology 244, 161–172. [DOI] [PubMed] [Google Scholar]
- 17.Yanagi, M., St. Claire, M., Emerson, S. U., Purcell, R. H. & Bukh, J. (1999) Proc. Natl. Acad. Sci. USA 96, 2291–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanagi, M., Purcell, R. H., Emerson, S. U. & Bukh, J. (1999) Virology 262, 250–263. [DOI] [PubMed] [Google Scholar]
- 19.Bukh, J., Pietschmann, T., Lohmann, V., Krieger, N., Faulk, K., Engle, R. E., Govindarajan, S., Shapiro, M., St. Claire, M. & Bartenschlager, R. (2002) Proc. Natl. Acad. Sci. USA 99, 14416–14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forns, X., Thimme, R., Govindarajan, S., Emerson, S. U., Purcell, R. H., Chisari, F. V. & Bukh, J. (2000) Proc. Natl. Acad. Sci. USA 97, 13318–13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanagi, M., Purcell, R. H., Emerson, S. U. & Bukh, J. (1997) Proc. Natl. Acad. Sci. USA 94, 8738–8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farci, P., Shimoda, A., Wong, D., Cabezon, T., De Gioannis, D., Strazzera, A., Shimizu, Y., Shapiro, M., Alter, H. J. & Purcell, R. H. (1996) Proc. Natl. Acad. Sci. USA 93, 15394–15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grakoui, A., Wychowski, C., Lin, C., Feinstone, S. M. & Rice, C. M. (1993) J. Virol. 67, 1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bukh, J., Apgar, C. L., Engle, R., Govindarajan, S., Hegerich, P. A., Tellier, R., Wong, D. C., Elkins, R. & Kew, M. C. (1998) J. Infect. Dis. 178, 1193–1197. [DOI] [PubMed] [Google Scholar]
- 25.Blight, K. J., Kolykhalov, A. A. & Rice, C. M. (2000) Science 290, 1972–1974. [DOI] [PubMed] [Google Scholar]
- 26.Tuplin, A., Wood, J., Evans, D. J., Patel, A. H. & Simmonds, P. (2002) RNA 8, 824–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer, W. B. & Sansom, M. S. (2002) Biochim. Biophys. Acta 1561, 27–45. [DOI] [PubMed] [Google Scholar]
- 28.Takeda, M., Pekosz, A., Shuck, K., Pinto, L. H. & Lamb, R. A. (2002) J. Virol. 76, 1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe, T., Watanabe, S., Ito, H., Kida, H. & Kawaoka, Y. (2001) J. Virol. 75, 5656–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma, C., Marassi, F. M., Jones, D. H., Straus, S. K., Bour, S., Strebel, K., Schubert, U., Oblatt-Montal, M., Montal, M. & Opella, S. J. (2002) Protein Sci. 11, 546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalinina, O., Norder, H., Mukomolov, S. & Magnius, L. O. (2002) J. Virol. 76, 4034–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.