Abstract
The developmental expression of macroscopic Ca2+-activated K+ currents (IK[Ca]) in chicken ciliary ganglion (CG) neurons is dependent in part on trophic factors released from preganglionic nerve terminals. Neuregulins are expressed in the preganglionic neurons that innervate the chicken CG and are therefore plausible candidates for this activity. Application of 1 nM β1-neuregulin peptide for 12 hr evokes a large (7- to 10-fold) increase in IK[Ca] in embryonic day 9 CG neurons, even in the presence of a translational inhibitor. A similar posttranslational effect is produced by high concentrations (10 nM) of epidermal growth factor and type α transforming growth factor but not by 10 nM α2-neuregulin peptide or by neurotrophins at 40 ng⋅ml−1. β1-neuregulin treatment for 12 hr also confers Ca2+ sensitivity onto large-conductance (285 pS) K+ channels observed in inside–out patches. β-Neuregulins have no effect on voltage-activated Ca2+ currents of CG neurons. These data support the hypothesis that β-neuregulins mediate the trophic effects of preganglionic nerve terminals on the electrophysiological differentiation of developing CG neurons.
The erbB family of receptors, also known as type 1 tyrosine kinase receptors, mediate the actions of many growth and differentiation factors. The erbB1 receptor can be activated by epidermal growth factor (EGF), type α transforming growth factor (TGFα), and several other factors (reviewed in refs. 1 and 2). The natural ligands for erbB3 and erbB4 receptors include the neuregulins, a large class of growth and differentiation factors expressed in several neural and mesenchymal tissues (3–6). Neuregulins do not bind to erbB1 receptors. Instead, they bind to erbB3 and erbB4 receptors, resulting in trans-activation of erbB2 (7–10) or erbB1 receptors (11). Activation of erbB1 receptors can also cause trans-activation of erbB2, but to date no ligands have been found that can cause direct activation of erbB2 in the absence of other receptor types.
Neuregulins regulate the development and differentiation of neural and mesenchymal tissues (reviewed in ref. 12). Many different neuregulin isoforms can be generated by alternative splicing (6, 12–16). All known isoforms can be divided into two distinct groups on the basis of differences in the structure of the EGF-like domains present in all neuregulins, as well as their biological activities (12, 16). Indeed, small fragments of these proteins that contain the EGF-like domains along with a few adjacent amino acid residues retain full biological activity (12). The various β-neuregulins are the predominant isoforms expressed in the nervous system (12, 14, 16). One of the most extensively studied actions of neuregulins is the stimulation of nicotinic acetylcholine receptor (AChR) expression at vertebrate neuromuscular junctions (5, 17, 18). This effect is caused by transcriptional activation of muscle subsynaptic nuclei (19–21), leading to the development and maintenance of a high density of AChRs at the endplate region. Neuregulins also stimulate the expression of voltage-activated Na+ channels in chicken myotubes (22), and may regulate neurogenesis in some sensory and autonomic ganglia (23, 24). In addition, a subset of β-neuregulins, known as glial growth factors, regulate the proliferation and differentiation of developing and mature glial cells and Schwann cells (6, 25, 26). In most neural systems described to date, β-neuregulins are more potent and efficacious than α-neuregulins and produce effects that are not mimicked by EGF or other erbB1 ligands (12, 16). However, some nonneuronal cells exhibit similar responses to neuregulins and erbB1 ligands (27).
Our previous studies have shown that the Ca2+-activated K+ current (IK[Ca]) is one of the largest ionic currents expressed in mature parasympathetic neurons of the chicken ciliary ganglion (CG) (28, 29). The normal developmental expression of whole-cell IK[Ca] in chicken autonomic neurons is dependent upon cell–cell interactions. For example, CG neurons that develop in vitro in the absence of other cell types fail to express a functional whole-cell IK[Ca] although voltage-activated Ca2+ currents are expressed at normal levels (29, 30). Moreover, a macroscopic IK[Ca] is not expressed at normal amplitude in CG neurons that develop in situ in the absence of interactions with either target tissues or preganglionic innervation, although voltage-activated Ca2+ currents are normal in both of those cases (31). The inductive effect of the afferent innervation is not due to synaptic activation of CG neurons as it persists when ganglionic transmission is blocked chronically in ovo (32). Therefore, the trophic effects of preganglionic innervation on CG IK[Ca] expression are mediated by a preganglionic nerve terminal-derived factor not directly related to cholinergic synaptic transmission per se. β-Neuregulins represent a plausible candidate for this activity because their transcripts are expressed in preganglionic Edinger–Westphal neurons that innervate the chicken CG (33). Moreover, β-neuregulins induce the rapid phosphorylation of erbB2 in chicken CG neurons (33). The functional effects of neuregulins and other erbB ligands in CG neurons are unknown. Therefore, the purpose of the present study was to examine the effects of erbB ligands on the functional expression of IK[Ca] in chicken CG neurons developing in vitro. The results support the hypothesis that β-neuregulins regulate the expression of whole-cell IK[Ca] in developing CG neurons.
MATERIALS AND METHODS
Cell Isolation and Culture.
Chicken CG neurons were isolated at embryonic day 9 (E9) and cultured as described previously in a medium containing 40 ng⋅ml−1 recombinant rat ciliary neurotrophic factor (CNTF) (29, 30, 34). Cells were maintained in vitro for 12 hr in the presence or absence of erbB ligands or other factors. In some early experiments, CG neurons were cultured in the presence or absence of neuregulins for 4 days. Similar results were obtained with those protocols. In some experiments, culture media contained 0.1 mg⋅ml−1 of the translational inhibitor anisomycin, a concentration that we have previously shown to inhibit more than 95% of protein synthesis in E9 CG neurons in vitro (30).
Electrophysiology.
Whole-cell recordings of IK[Ca] were made using standard methods as described previously (28–32). Briefly, 25-ms depolarizing steps to 0 mV were applied from a holding potential of −40 mV. Normal external saline contained 145 mM NaCl/5.4 mM KCl/5.4 mM CaCl2/0.8 mM MgCl2/5 mM d-glucose/13 mM Hepes/500 nM tetrodotoxin, pH 7.4. Nominally Ca2+-free external salines were the same except that they contained 5.8 mM MgCl2 and no added CaCl2. Pipette solutions contained 120 mM KCl/2 mM MgCl2/10 mM EGTA/5 mM ATP/0.2 mM GTP/0.1 mM leupeptin, pH 7.4. For experiments on voltage-activated Ca2+ currents, KCl in the pipette saline was replaced with CsCl. Currents were evoked in normal and Ca2+-free salines, and the net Ca2+-dependent currents were obtained by digital subtraction. Currents were normalized for cell size by computing soma surface area for each cell from its diameter as measured with an ocular micrometer (29, 30, 35, 36). Data were analyzed by one-way ANOVA when multiple comparisons were made, followed by Bonferonni’s t-test when significant differences were observed between groups. Student’s unpaired t-test was used when each group had its own control. Throughout, P < 0.05 was regarded as significant. Single-channel analyses of IK[Ca] in excised inside–out patches were performed as described previously (28, 37). Pipette salines contained 150 mM KCl/5 mM Hepes/10 mM EGTA, pH 7.4. Bath salines that contained no free Ca2+ consisted of 75 mM NaCl/75 mM KCl/5 mM Hepes/5 mM EGTA, pH 7.4. A second saline was the same except that 5 mM CaCl2 was added, resulting in a free Ca2+ concentration of approximately 10 μM (28). Data were filtered at 5 kHz with a four-pole Bessel filter and stored on magnetic videotape for later off-line analysis using pclamp6.03 software. Data were digitized at 25 kHz and events were idealized using a half-threshold crossing procedure that ignored transitions of less than 300 μs duration.
Neuregulins and Other Trophic Factors.
Recombinant neuregulin peptides were generously provided by G. D. Fischbach of the Harvard Medical School (Boston). These consisted of a β1-neuregulin peptide (amino acids 177–246 of human β1-neuregulin) and an α2-neuregulin peptide (consisting of amino acids 19–241 of human α2-neuregulin). These neuregulin peptides are biologically active in several systems (12). In a few experiments we also examined a full-length recombinant chicken β-neuregulin known as pro-ARIA (5). Recombinant human TGFα was obtained from Calbiochem. Recombinant rat neurotrophins, recombinant rat CNTF, and recombinant human EGF were obtained from BioSource International, Camarillo, CA.
RESULTS
CG neurons were isolated at E9 and cultured for 12 hr in the presence and absence of 1 nM recombinant human β1-neuregulin peptide (β-NEU). Control CG neurons usually expressed detectable macroscopic IK[Ca] but this current was small, with a mean current density of less than 0.02 mA⋅cm−2 (Fig. 1 A and C). β-NEU treatment for 12 hr caused a robust (5- to 7-fold) stimulation of whole-cell IK[Ca] compared with control neurons (Fig. 1 B and C) but had no effect on the amplitude or density of voltage-activated Ca2+ currents observed with CsCl recording pipettes (Fig. 2). IK[Ca] density in β-NEU-treated E9 neurons is comparable to that observed in acutely isolated E13 CG neurons (29, 30). A similar effect was produced by exposure of CG neurons for 12 hr to a full-length recombinant chicken β1-neuregulin protein, but β-neuregulin treatment for 2 hr did not result in significant stimulation of IK[Ca] (data not shown). By contrast, α2-neuregulin peptide (α-NEU) was ineffective even at 10 nM (Fig. 1C). The stimulatory effects of β-NEU were posttranslational (Fig. 1D), as they persisted in the presence of the translational inhibitor anisomycin (0.1 mg⋅ml−1), a concentration that we have previously shown to block more than 95% of protein synthesis in E9 CG neurons (30). Therefore, the effects of neuregulins are exerted on preexisting IK[Ca] channels.
Figure 1.
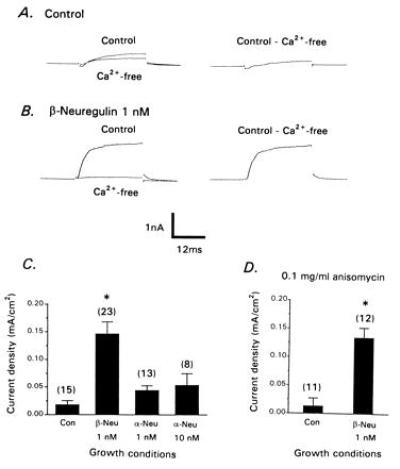
β-Neuregulins stimulate whole-cell IK[Ca]. CG neurons were isolated at E9 and cultured for 12 hr in the absence (A) or presence (B) of 1 nM β-neuregulin peptide. Whole-cell currents were evoked by depolarizing steps to 0 mV from a holding potential of −40 mV in the presence and absence of Ca2+ as indicated (Left), and net Ca2+-dependent currents were obtained by digital subtraction (Right). (C) Density of net Ca2+-dependent outward currents in CG neurons that developed in the presence or absence of β-neuregulin or α-neuregulin peptides as indicated. (D) The stimulatory effects of β-neuregulin peptide persist in the presence of the translational inhibitor anisomycin. In this and subsequent figures, the number of cells per group is indicated in parentheses above each bar, error bars represent SEM, and asterisks indicate significant (P < 0.05) difference from controls.
Figure 2.
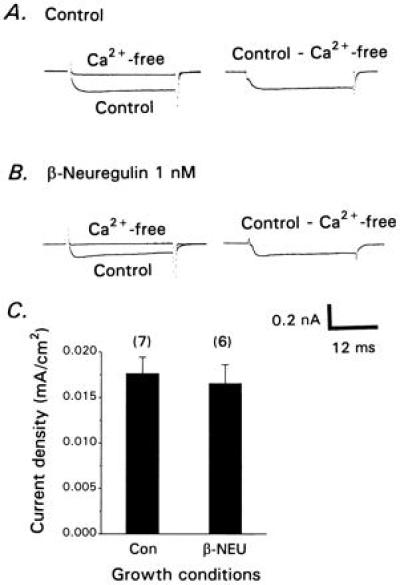
β-Neuregulin treatment has no effect on voltage-activated Ca2+ currents of CG neurons. Cells were isolated at E9 and cultured for 12 hr in the absence (A) or presence (B) of 1 nM β-neuregulin peptide. Recordings were made as described in Fig. 1 except that recording pipettes contained CsCl. Currents were evoked in the presence and absence of external Ca2+ (Left), and net voltage-activated Ca2+ currents were then obtained by digital subtraction (Right). (C) Mean Ca2+ current density is not affected by β-neuregulin treatment.
Other erbB ligands could also stimulate whole-cell IK[Ca], but only at relatively high concentrations (Fig. 3A). For example, 12-hr application of 10 nM EGF or 10 nM TGFα caused a 5- to 7-fold stimulation of IK[Ca]. However, these factors did not produce significant stimulation of IK[Ca] at 1 nM (data not shown). As with β-NEU, the stimulatory effects of 10 nM EGF and 10 nM TGFα can be evoked in the continued presence of 0.1 mg/ml anisomycin and therefore do not require synthesis of new IK[Ca] channel proteins (Fig. 3B). Other factors tested, including four different neurotrophins (nerve growth factor, recombinant rat brain-derived neurotrophic factor, and recombinant rat neurotrophin-3 and 4), were inactive even at high concentrations (Fig. 3C).
Figure 3.
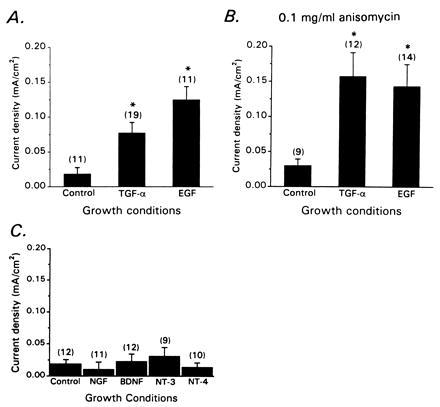
Effects of erbB1 ligands and neurotrophins on whole-cell IK[Ca]. (A) Stimulatory effects of 12-hr treatment with 10 nM EGF and TGFα on CG neurons isolated at E9. (B) The stimulatory effects of these factors persist in the continued presence of 0.1 mg/ml anisomycin. (C) Application of neurotrophins (40 ng/ml) for 12 hr has no effect on whole-cell IK[Ca] of chicken CG neurons.
The experiments described above indicate that erbB ligands regulate macroscopic IK[Ca] by posttranslational mechanisms, but they do not indicate whether this effect is exerted directly on the IK[Ca] channels. For example, stimulation of IK[Ca] could have been produced by modulating the functional coupling of IK[Ca] and voltage-activated Ca2+ channels (38) or even by altering the binding or sequestration of intracellular free Ca2+ (39). Therefore, we examined the properties of single IK[Ca] channels in inside–out patches excised from E9 CG neurons grown for 12 hr in the presence and absence of 1 nM β-NEU. If neuregulins were acting primarily by altering Ca2+ channel localization or intracellular Ca2+ handling then one would expect to see normal IK[Ca] channels in excised inside–out patches from control E9 neurons (because the Ca2+ is provided in the bath saline). That was not observed (Fig. 4A). Instead, the majority of patches from control neurons contained 285-pS K+ channels that exhibited a moderate level of activity at −60 mV, even in Ca2+-free salines. These channels reversed very close to the calculated K+ equilibrium potential (EK) (Fig. 4A). The mean probability of channel opening (Po) of the 285-pS K+ channels observed at −60 mV in Ca2+-free bath saline was 0.117 ± .032 (mean ± SEM, n = 7 patches). These channels occasionally exhibited a slight increase in Po after application of a bath saline containing 10 μM free Ca2+ (Po = 0.158 ± .055), but this was never as great as is seen in acutely isolated E13 neurons (28). Very different behavior was observed in E9 CG neurons cultured for 12 hr in the presence of 1 nM β-NEU (Fig. 4B). Patches excised from β-NEU-treated cells were essentially quiescent at −60 mV in Ca2+-free bath salines. Under those conditions the mean Po of large-conductance K+ channels was 0.012 ± .007 (n = 8 patches). Upon exposure to 10 μM Ca2+ the majority of these patches exhibited a large increase in the activity of a 285-pS K+ channel (to a mean Po of 0.289 ± .056). These unitary currents reversed at the calculated EK (Fig. 4B). In a few patches, these very large-conductance channels were accompanied by 185 and 110 pS K+ channels (not shown). The percentage increase in Po evoked by 10 μM Ca2+ and the steady-state Po under those conditions were significantly (P < 0.05) greater in β-NEU-treated cells than in control cells. One aspect of these results was surprising. The IK[Ca] channels in acutely isolated E13 CG neurons typically have unitary conductances of 45, 110, and 185 pS, and we have never seen 285 pS IK[Ca] channels at E13 (28, 37). A more detailed description of the biophysical and pharmacological properties of the 285-pS channels of E9 CG neurons will be the subject of another report. Here we conclude only that β-neuregulin treatment results in profound changes in the behavior of large-conductance IK[Ca] channels. Note also that developmental changes in the Ca2+ dependence of large-conductance K+ channels have been reported in other vertebrate neurons (40, 41).
Figure 4.
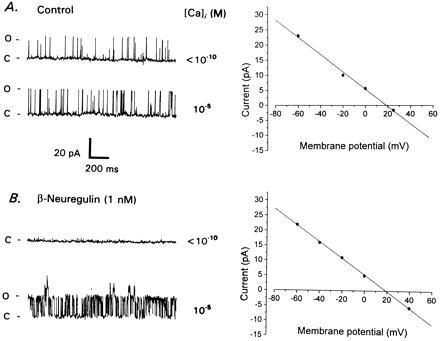
Effects of β-neuregulin on large-conductance K+ channels in developing CG neurons. (A) Inside–out patch recording from E9 neuron cultured for 12 hr in the absence of trophic factor. Traces to the left show large-conductance K+ channels in Ca2+-free saline (Upper Left) and in saline with free Ca2+ buffered to approximately 10 μM (Lower Left). Membrane potential is −60 mV. Note that gating of large-conductance channels is essentially independent of free Ca2+ concentration. These large-conductance channels reverse close to the calculated EK (+17 mV) and have a unitary slope conductance of 285 pS (Right). (B) Inside–out patch from E9 CG neuron cultured for 12 hr with 1 nM β-neuregulin peptide. Patch is quiescent in Ca2+-free saline (Upper Left), but large-conductance channels exhibit high level of activity in the presence of saline containing 10 μM free Ca2+ (Lower Left). Membrane potential is −60 mV. These large-conductance channels reverse close to calculated EK and have a unitary slope conductance of 285 pS (Right).
DISCUSSION
In the present study, we have observed that β-neuregulins modulate the functional expression of IK[Ca] in chicken CG neurons developing in vitro. Similar effects were produced by high concentrations of EGF and TGFα, but not by high concentrations of α2-neuregulin peptide or neurotrophins. The developmental expression of whole-cell IK[Ca] in situ is dependent on inductive interactions with other cell types, including target tissues in the eye and the afferent preganglionic innervation (31). β-Neuregulin transcripts are expressed in the preganglionic neurons that innervate the chicken CG (33), and it is likely that developing CG neurons are exposed to neuregulins secreted from preganglionic nerve terminals. Moreover, the trophic effect of the preganglionic innervation appears to be mediated by a secreted factor unrelated to synaptic transmission per se (32). Therefore, it is reasonable to hypothesize that β-neuregulins play a role in mediating the trophic effect of preganglionic nerve terminals on the functional expression of IK[Ca]. The results of the present study support this hypothesis, but it should be noted that the normal developmental expression of functional IK[Ca] channels in vivo is a complex process that also requires interactions with target-derived factors (30, 31).
To our knowledge, this is the first report of an effect of neuregulins on neuronal function mediated by posttranslational mechanisms. Previous studies have shown that neuregulins cause transcriptional activation of target cells, such as developing vertebrate myotubes (19, 20, 21). This suggests that activation of erbB2 receptors causes a cascade of effects specific for a given cell type. In this regard, we have noted that β-NEU fails to stimulate IK[Ca] in developing chicken lumbar sympathetic ganglion neurons, which express erbB2 receptors that can be phosphorylated in response to β-neuregulins (33) as well as an IK[Ca] whose expression is regulated by soluble differentiation factors (36). Thus, neuregulins resemble the neurotrophins in that they produce a number of cell type-specific actions exerted over a wide range of time scales (42).
The physiological significance of the IK[Ca] stimulatory activity of the erbB1 ligands in CG neurons is not clear. High concentrations were required, well above those required to see physiologically significant effects in other systems. Nevertheless, this result is of interest because even high concentrations of EGF are unable to stimulate AChR synthesis in myotubes. We have recently reported that a soluble 40- to 60-kDa factor expressed in the iris, one of the principal target tissues of the CG, can stimulate whole-cell IK[Ca] in CG neurons by posttranslational mechanisms (30). The 40- to 60-kDa fractions obtained from the iris do not stimulate AChR expression in cultured myotubes and do not contain immunochemically detectable neuregulins (A. Goodearle and S.E.D., unpublished observations). One possibility is that the target-derived factor is an erbB1 ligand or a related molecule. It is also possible that the target-derived IK[Ca] stimulatory factor is structurally unrelated to EGF but that it activates signaling pathways that converge at some point with those activated by erbB ligands.
The mechanism of β-neuregulin modulation of IK[Ca] in CG neurons is unknown. However, it should be noted that the response to neuregulins in CG neurons is relatively slow for a posttranslational event, requiring several hours to see a significant stimulation of IK[Ca]. This suggests that several enzymatic steps precede modification of the channels. The nature of the modification of IK[Ca] channels is also unknown, but may entail direct phosphorylation or dephosphorylation of the channel molecules or closely associated subunits. There is a large literature indicating that phosphorylation can increase (43) or decrease (43, 44) the activity of large-conductance IK[Ca] channels and that protein kinases and phosphatases are closely associated with IK[Ca] channels in neuronal membranes (45). The present data indicate that β-neuregulins induce modifications of preexisting IK[Ca] channels consistent with the observed changes in the macroscopic currents. However, they do not exclude that β-neuregulins may also exert subtle effects on Ca2+ binding, sequestration, or availability to activate IK[Ca] channels. The unitary conductance of IK[Ca] channels observed in E9 neurons is greater than that observed in E13 cells. This raises the possibility of a developmental change in the subunit composition of IK[Ca] channels on CG neurons, possibly programmed internally. Large-conductance IK[Ca] channels are encoded at a single locus known as slowpoke (slo), but this gene can be expressed in many different variants as a result of alternative splicing (46, 47) and alternative promoter utilization (48). We have previously shown that multiple slo transcripts are expressed in chicken CG neurons (30), but we know nothing about the subunit stoichiometry of the functional channels or how this might change during embryonic development.
In summary, we have shown that whole-cell IK[Ca] in developing parasympathetic neurons can be stimulated by β-neuregulins and by high concentrations of structurally related erbB ligands. This effect is exerted on preexisting IK[Ca] channels, which may not be identical to those found at later developmental stages.
Acknowledgments
This paper is dedicated to the memory of our colleague, Dr. R. Bruce Masterton. We are grateful to Dr. Gerald Fischbach of the Harvard Medical School for supplying neuregulin peptides. Additionally, we are grateful to Michelle Reiser for expert technical assistance and to Drs. Ken Rosen and Andrew Goodearle for helpful discussions. This paper was supported by National Institutes of Health Grant NS-32748 (S.E.D.) and a Graduate Student Fellowship from the American Heart Association (P.S.).
ABBREVIATIONS
- CG
chicken ciliary ganglion
- IK[Ca]
Ca2+-activated K+ current
- β-NEU
β-1 neuregulin peptide
- α-NEU
α-2 neuregulin peptide
- TGFα
type α recombinant human transforming growth factor
- EGF
recombinant human epidermal growth factor
- AChR
acetylcholine receptor
References
- 1.Schlessinger J, Ullrich A. Neuron. 1992;9:383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- 2.Massague J, Pandiella A. Annu Rev Biochem. 1993;62:515–541. doi: 10.1146/annurev.bi.62.070193.002503. [DOI] [PubMed] [Google Scholar]
- 3.Wen D, Peles E, Cupples R, Suggs S V, Bacus S S, Luo Y, Trail G, Hu S, Silbiger S M, Ben Levy R, Koski R A, Lu H S, Yarden Y. Cell. 1992;69:559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- 4.Holmes W E, Sliwkowski M X, Akita R W, Henzel W J, Lee J, Park J W, Yensura D, Absdi N, Raab H, Lewis G D, Shepard H, Kuang W-J, Wood W I, Goedel D V, Vandlen R L. Science. 1992;256:1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- 5.Falls D L, Rosen K M, Corfas G, Lane W S, Fischbach G D. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- 6.Marchionni M A, Goodearle A D J, Maio S C, Berningham-McDonogh O, Kirk C, et al. Nature (London) 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 7.Plowman G D, Culouscou J M, Whitney G S, Green J M, Carlton G W, Foy L, Neubaurer M G, Shoyab M. Proc Natl Acad Sci USA. 1993;90:1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plowman G D, Green J M, Culouscou J M, Carlton G W, Rothwell V M, Buckley S. Nature (London) 1993;366:473–475. doi: 10.1038/366473a0. [DOI] [PubMed] [Google Scholar]
- 9.Carraway K L, Cantley L C. Cell. 1994;78:5–8. doi: 10.1016/0092-8674(94)90564-9. [DOI] [PubMed] [Google Scholar]
- 10.Sliwkowski M X, Schaefer G, Akita R W, Lofgren J A, Fitzpatrick V D, Nuijens A, Fendly B M, Ceruibe R A, Vandlen R L, Carraway K L. J Biol Chem. 1994;269:14661–14665. [PubMed] [Google Scholar]
- 11.Riese D J, van Raaij T M, Plowman G D, Andrews G C, Stern D F. Mol Cell Biol. 1995;15:5770–5776. doi: 10.1128/mcb.15.10.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischbach, G. D. & Rosen, K. M. (1997) Annu. Rev. Neurosci., in press. [DOI] [PubMed]
- 13.Orr-Urtreger A, Trakhtenbrot L, Ben-Levy R, Wen D, Rechavi G, Lonai P, Yarden Y. Proc Natl Acad Sci USA. 1993;90:1867–1871. doi: 10.1073/pnas.90.5.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer D, Birchmeyer C. Proc Natl Acad Sci USA. 1994;91:1064–1068. doi: 10.1073/pnas.91.3.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Baruch N, Yarden Y. Proc Soc Exp Biol Med. 1994;206:221–227. doi: 10.3181/00379727-206-43746. [DOI] [PubMed] [Google Scholar]
- 16.Wen D, Suggs S V, Karunagaran D, Liu N, Cupples R L, Luo Y, Janssen A M, Ben-Baruch N, Trollinger D B, Jacobsen V L, Meng S Y, Lu H S, Hu S, Chang D, Yang W N, Yanigahara D, Koski R A, Yarden Y. Mol Cell Biol. 1994;14:1909–1919. doi: 10.1128/mcb.14.3.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corfas G, Falls D L, Fischbach G D. Proc Natl Acad Sci USA. 1993;90:1624–1628. doi: 10.1073/pnas.90.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moscoso L M, Chu G C, Gautam M, Noakes P G, Merlie J P, Sanes J R. Dev Biol. 1995;172:158–169. doi: 10.1006/dbio.1995.0012. [DOI] [PubMed] [Google Scholar]
- 19.Harris D A, Falls D L, Dill-Devor R M, Fischbach G D. Proc Natl Acad Sci USA. 1988;85:1983–1987. doi: 10.1073/pnas.85.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinou J C, Falls D L, Fischbach G D, Merlie J P. Proc Natl Acad Sci USA. 1991;88:7669–7673. doi: 10.1073/pnas.88.17.7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu G C, Moscoso L M, Sliwkowski M X, Merlie J P. Neuron. 1995;14:329–339. doi: 10.1016/0896-6273(95)90289-9. [DOI] [PubMed] [Google Scholar]
- 22.Corfas G, Fischbach G D. J Neurosci. 1993;13:2118–2125. doi: 10.1523/JNEUROSCI.13-05-02118.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer R, Bucay N, Kane D J, Martin L E, Tarpley J E, Theill L E. Proc Natl Acad Sci USA. 1996;93:4833–4838. doi: 10.1073/pnas.93.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer D, Birchmeyer C. Nature (London) 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 25.Morrissey T K, Levi A D, Nuijens A, Sliwkowski M X. Proc Natl Acad Sci USA. 1995;92:1431–1435. doi: 10.1073/pnas.92.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen K R. Neuron. 1995;15:585–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 27.Gamett D C, Greente T, Wasreich A R, Kim H H, Koland J G, Creione R A. J Biol Chem. 1995;270:19022–19027. doi: 10.1074/jbc.270.32.19022. [DOI] [PubMed] [Google Scholar]
- 28.Dryer S E, Dourado M M, Wisgirda M E. J Physiol (London) 1991;443:601–627. doi: 10.1113/jphysiol.1991.sp018854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dourado M M, Dryer S E. J Physiol (London) 1992;449:411–428. doi: 10.1113/jphysiol.1992.sp019093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramony P, Raucher S, Dryer L, Dryer S E. Neuron. 1996;17:115–124. doi: 10.1016/s0896-6273(00)80285-8. [DOI] [PubMed] [Google Scholar]
- 31.Dourado M, Wisgirda M, Jacob M H, Dryer S E. J Neurosci. 1994;14:3156–3165. doi: 10.1523/JNEUROSCI.14-05-03156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramony P, Dryer S E. Dev Brain Res. 1996;91:149–152. doi: 10.1016/0165-3806(95)00174-3. [DOI] [PubMed] [Google Scholar]
- 33.Corfas G, Rosen K M, Aratake H, Krauss R, Fischbach G D. Neuron. 1995;14:103–115. doi: 10.1016/0896-6273(95)90244-9. [DOI] [PubMed] [Google Scholar]
- 34.Dourado M, Dryer S E. J Physiol (London) 1994;474:367–377. doi: 10.1113/jphysiol.1994.sp020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raucher S, Dryer S E. J Physiol (London) 1994;479:77–94. doi: 10.1113/jphysiol.1994.sp020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raucher S, Dryer S E. J Physiol (London) 1995;486:605–614. doi: 10.1113/jphysiol.1995.sp020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dryer S E. J Physiol (London) 1991;435:513–532. doi: 10.1113/jphysiol.1991.sp018522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wisgirda M E, Dryer S E. Proc Natl Acad Sci USA. 1994;91:2858–2862. doi: 10.1073/pnas.91.7.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meech R W, Standen N B. J Physiol (London) 1975;249:211–259. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blair L A C, Dionne V E. Nature (London) 1985;315:229–131. doi: 10.1038/315329a0. [DOI] [PubMed] [Google Scholar]
- 41.Mienville J-M, Barker J L. Pflügers Arch. 1995;431:763–770. doi: 10.1007/BF02253841. [DOI] [PubMed] [Google Scholar]
- 42.Lo D. Neuron. 1995;15:979–981. doi: 10.1016/0896-6273(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 43.Reinhart P H, Chung S K, Martin B L, Brautigan D L, Levitan I B. J Neurosci. 1991;11:16727–1635. doi: 10.1523/JNEUROSCI.11-06-01627.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White R E, Schonbrunn A, Armstrong D L. Nature (London) 1991;351:570–573. doi: 10.1038/351570a0. [DOI] [PubMed] [Google Scholar]
- 45.Chung S K, Reinhart P H, Martin B L, Brautigan D L, Levitan I B. Science. 1991;253:560–562. doi: 10.1126/science.1857986. [DOI] [PubMed] [Google Scholar]
- 46.Butler A B, Tsunoda S, McCobb D, Wei A, Salkoff L. Science. 1993;261:221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- 47.Tseng-Crank J, Foster C D, Krause J D, Mertz R, Godinot N, DiChiara T J, Reinhart P H. Neuron. 1994;13:1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 48.Brenner R, Thomas T, Atkinson N. J Neurosci. 1996;16:1827–1835. doi: 10.1523/JNEUROSCI.16-05-01827.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


