Abstract
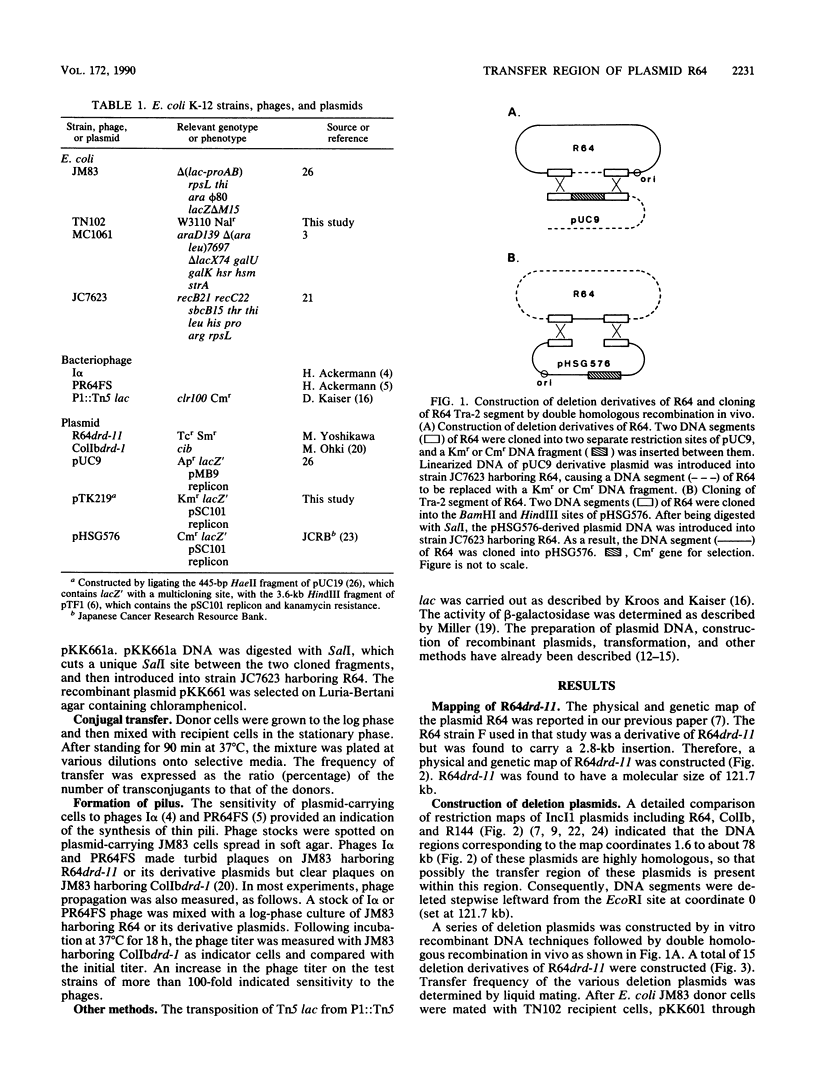
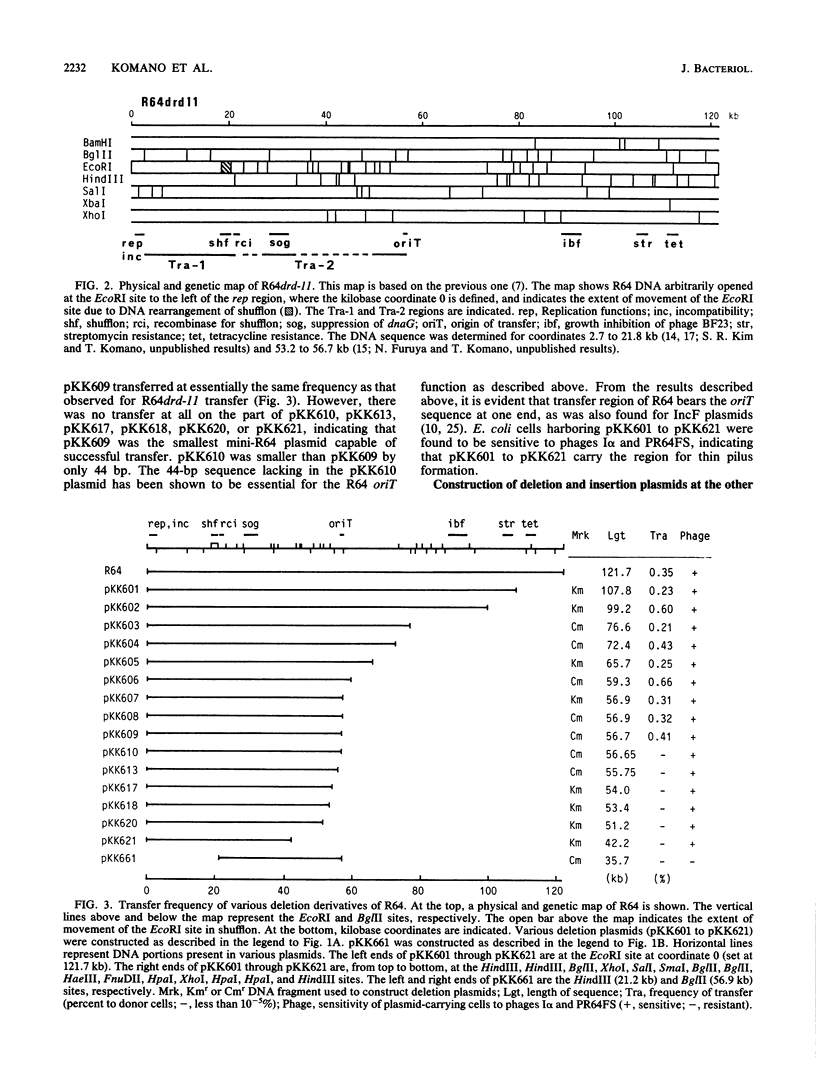
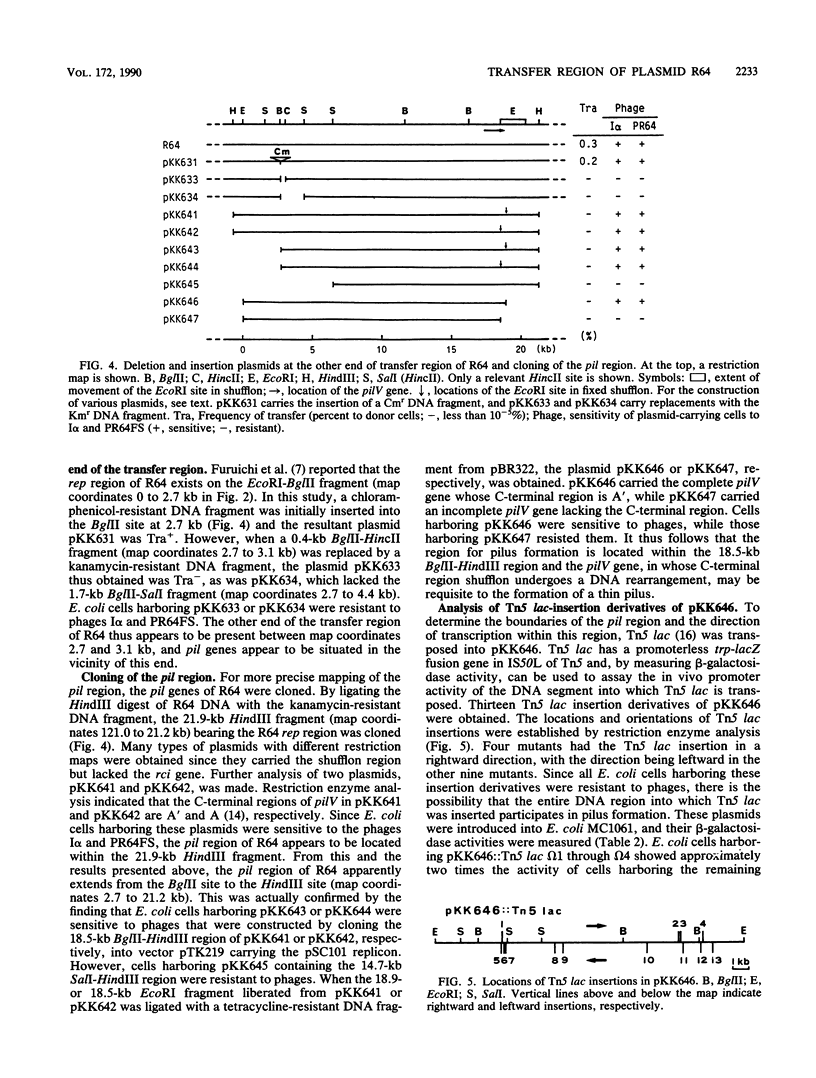
To locate the transfer region of the 122-kiloase plasmid R64drd-11 belonging to incompatibility group I1, a series of deletion derivatives was constructed by in vitro recombinant DNA techniques followed by double homologous recombination in vivo. A plasmid designated pKK609 and bearing a 56.7-kilobase R64 sequence was the smallest transferable plasmid. A plasmid designated pKK610 and no longer possessing the 44-base-pair sequence of the R64 transfer system is located at one end. The other end of the R64 transfer region comprises a DNA segment of about 19 kilobases responsible for pilus formation. Shufflon, DNA with a novel rearrangement in R64, was found to be involved in pilus formation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. E. Characteristics and function of thick and thin conjugative pili determined by transfer-derepressed plasmids of incompatibility groups I1, I2, I5, B, K and Z. J Gen Microbiol. 1984 Jun;130(6):1489–1502. doi: 10.1099/00221287-130-6-1489. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Derepressed plasmids of incompatibility group I1 determine two different morphological forms of pilus. Plasmid. 1983 May;9(3):331–334. doi: 10.1016/0147-619x(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Coetzee J. N., Bradley D. E., Hedges R. W. Phages I alpha and I2-2: IncI plasmid-dependent bacteriophages. J Gen Microbiol. 1982 Nov;128(11):2797–2804. doi: 10.1099/00221287-128-11-2797. [DOI] [PubMed] [Google Scholar]
- Coetzee J. N., Sirgel F. A., Lecatsas G. Properties of a filamentous phage which adsorbs to pili coded by plasmids of the IncI complex. J Gen Microbiol. 1980 Apr;117(2):547–551. doi: 10.1099/00221287-117-2-547. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Inouye M., Inouye S. Novel one-step cloning vector with a transposable element: application to the Myxococcus xanthus genome. J Bacteriol. 1985 Oct;164(1):270–275. doi: 10.1128/jb.164.1.270-275.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi T., Komano T., Nisioka T. Physical and genetic analyses of the Inc-I alpha plasmid R64. J Bacteriol. 1984 Jun;158(3):997–1004. doi: 10.1128/jb.158.3.997-1004.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartskeerl R. A., Bergmans J. E., Kamp M. C., Hoekstra W. P. Cloning of an exclusion-determining fragment of the IncI plasmid, R144. Plasmid. 1983 Jul;10(1):11–20. doi: 10.1016/0147-619x(83)90053-7. [DOI] [PubMed] [Google Scholar]
- Hartskeerl R. A., vd Guchte M., Zuidweg E. M., Hoekstra W. P. Physical and genetic characterization of the IncI plasmid R144-drd3. Plasmid. 1984 Nov;12(3):215–217. doi: 10.1016/0147-619x(84)90048-9. [DOI] [PubMed] [Google Scholar]
- Ippen-Ihler K. A., Minkley E. G., Jr The conjugation system of F, the fertility factor of Escherichia coli. Annu Rev Genet. 1986;20:593–624. doi: 10.1146/annurev.ge.20.120186.003113. [DOI] [PubMed] [Google Scholar]
- Komano T., Furuichi T., Teintze M., Inouye M., Inouye S. Effects of deletion of the gene for the development-specific protein S on differentiation in Myxococcus xanthus. J Bacteriol. 1984 Jun;158(3):1195–1197. doi: 10.1128/jb.158.3.1195-1197.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano T., Kim S. R., Nisioka T. Distribution of shufflon among IncI plasmids. J Bacteriol. 1987 Nov;169(11):5317–5319. doi: 10.1128/jb.169.11.5317-5319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano T., Kubo A., Kayanuma T., Furuichi T., Nisioka T. Highly mobile DNA segment of IncI alpha plasmid R64: a clustered inversion region. J Bacteriol. 1986 Jan;165(1):94–100. doi: 10.1128/jb.165.1.94-100.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano T., Kubo A., Nisioka T. Shufflon: multi-inversion of four contiguous DNA segments of plasmid R64 creates seven different open reading frames. Nucleic Acids Res. 1987 Feb 11;15(3):1165–1172. doi: 10.1093/nar/15.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano T., Toyoshima A., Morita K., Nisioka T. Cloning and nucleotide sequence of the oriT region of the IncI1 plasmid R64. J Bacteriol. 1988 Sep;170(9):4385–4387. doi: 10.1128/jb.170.9.4385-4387.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroos L., Kaiser D. Construction of Tn5 lac, a transposon that fuses lacZ expression to exogenous promoters, and its introduction into Myxococcus xanthus. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5816–5820. doi: 10.1073/pnas.81.18.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A., Kusukawa A., Komano T. Nucleotide sequence of the rci gene encoding shufflon-specific DNA recombinase in the IncI1 plasmid R64: homology to the site-specific recombinases of integrase family. Mol Gen Genet. 1988 Jul;213(1):30–35. doi: 10.1007/BF00333394. [DOI] [PubMed] [Google Scholar]
- Merryweather A., Rees C. E., Smith N. M., Wilkins B. M. Role of sog polypeptides specified by plasmid ColIb-P9 and their transfer between conjugating bacteria. EMBO J. 1986 Nov;5(11):3007–3012. doi: 10.1002/j.1460-2075.1986.tb04599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki M., Ozeki H. Isolation of conjugation-constitutive mutants of colicin factor Ib. Mol Gen Genet. 1968;103(1):37–41. doi: 10.1007/BF00271155. [DOI] [PubMed] [Google Scholar]
- Oishi M., Cosloy S. D. The genetic and biochemical basis of the transformability of Escherichia coli K12. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1568–1572. doi: 10.1016/0006-291x(72)90520-7. [DOI] [PubMed] [Google Scholar]
- Rees C. E., Bradley D. E., Wilkins B. M. Organization and regulation of the conjugation genes of IncI1 plasmid colIb-P9. Plasmid. 1987 Nov;18(3):223–236. doi: 10.1016/0147-619x(87)90065-5. [DOI] [PubMed] [Google Scholar]
- Takeshita S., Sato M., Toba M., Masahashi W., Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61(1):63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- Willetts N., Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev. 1984 Mar;48(1):24–41. doi: 10.1128/mr.48.1.24-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


