Abstract
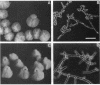
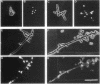
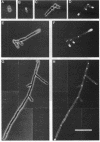
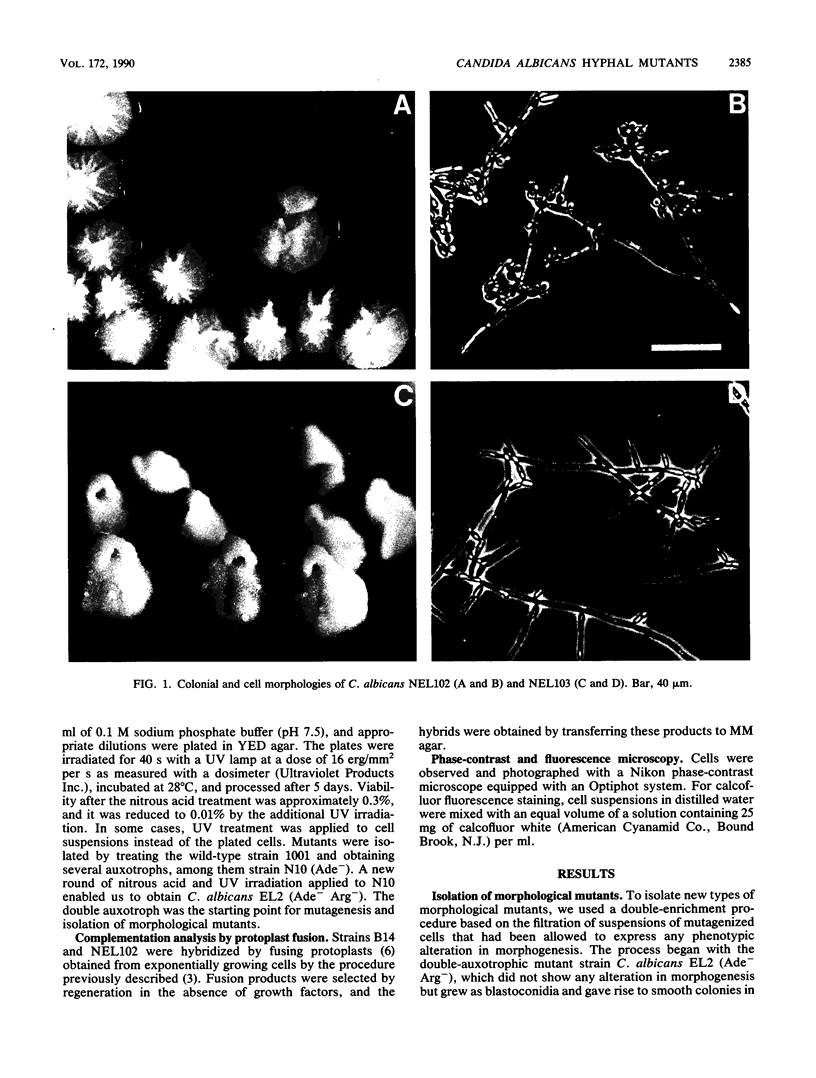
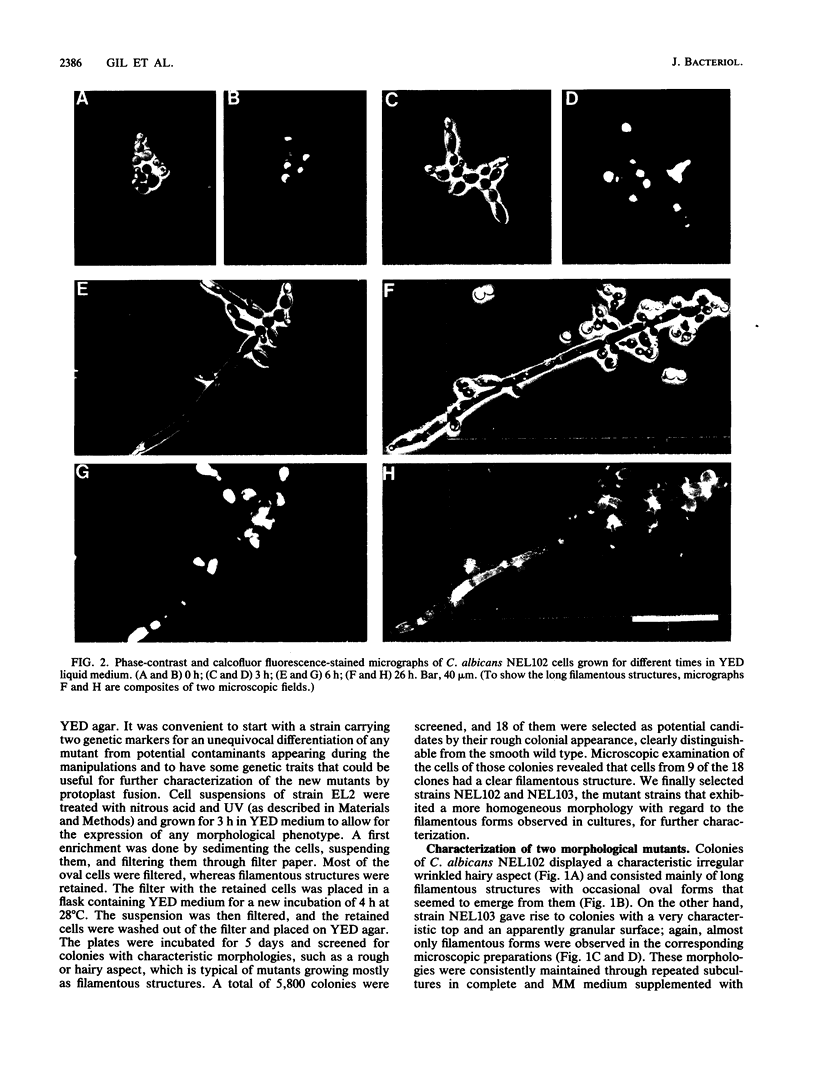
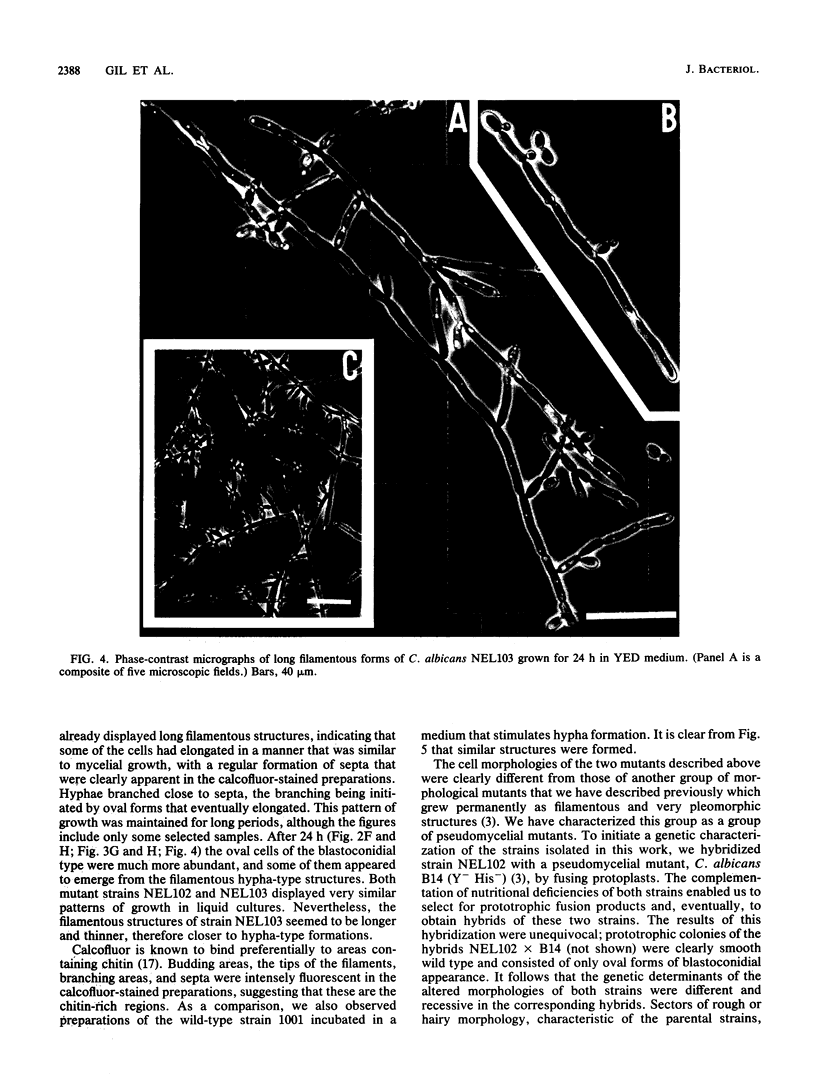
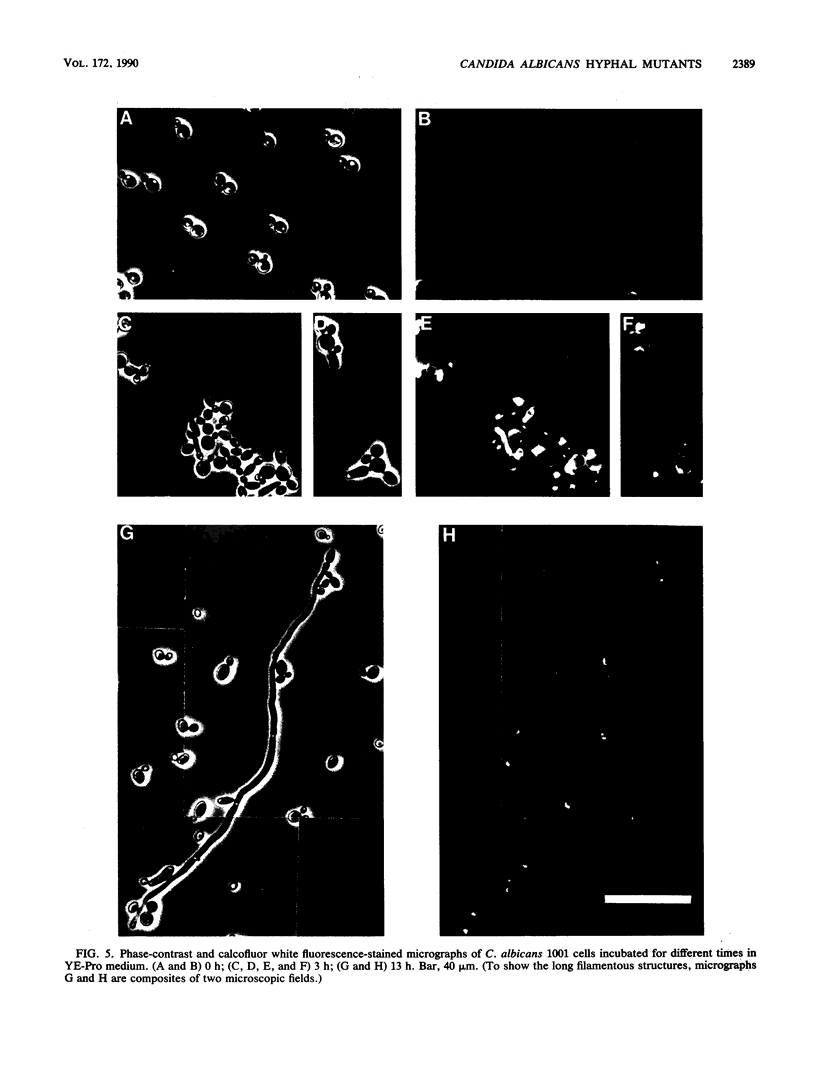
Several Candida albicans morphological mutants were obtained by a procedure based on a combined treatment with nitrous acid plus UV irradiation and a double-enrichment step to increase the proportion of mutants growing as long filamentous structures. Altered cell morphogenesis in these mutants correlated with an altered colonial phenotype. Two of these mutants, C. albicans NEL102 and NEL103, were selected and characterized. Mutant blastoconidia initiated budding but eventually gave rise to filamentous hypha-type formations. These filaments were long and septate, and they branched very regularly at positions near septa. Calcofluor white (which is known to bind chitin-rich areas) stained septa, branching zones, and filament tips very intensely, as observed under the fluorescence microscope. Wild-type hybrids were obtained by fusing protoplasts of strain NEL102 with B14, another morphological mutant previously described as being permanently pseudomycelial, indicating that genetic determinants responsible for the two altered phenotypes are different. The mutants characterized in this work seemed to sequentially express the morphogenic characteristics of C. albicans, from blastoconidia to hyphae, in the absence of any inducer. Further characterization of these strains could be relevant to gain understanding of the genetic control of dimorphism in this species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cannon R. D. Isolation of a mycelial mutant of Candida albicans. J Gen Microbiol. 1986 Aug;132(8):2405–2407. doi: 10.1099/00221287-132-8-2405. [DOI] [PubMed] [Google Scholar]
- Casanova M., Gil M. L., Cardeñoso L., Martinez J. P., Sentandreu R. Identification of wall-specific antigens synthesized during germ tube formation by Candida albicans. Infect Immun. 1989 Jan;57(1):262–271. doi: 10.1128/iai.57.1.262-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defever K. S., Whelan W. L., Rogers A. L., Beneke E. S., Veselenak J. M., Soll D. R. Candida albicans resistance to 5-fluorocytosine: frequency of partially resistant strains among clinical isolates. Antimicrob Agents Chemother. 1982 Nov;22(5):810–815. doi: 10.1128/aac.22.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil C., Pomés R., Nombela C. A complementation analysis by parasexual recombination of Candida albicans morphological mutants. J Gen Microbiol. 1988 Jun;134(6):1587–1595. doi: 10.1099/00221287-134-6-1587. [DOI] [PubMed] [Google Scholar]
- Gow N. A., Gooday G. W. A model for the germ tube formation and mycelial growth form of Candida albicans. Sabouraudia. 1984;22(2):137–144. doi: 10.1080/00362178485380211. [DOI] [PubMed] [Google Scholar]
- Hubbard M. J., Markie D., Poulter R. T. Isolation and morphological characterization of a mycelial mutant of Candida albicans. J Bacteriol. 1986 Jan;165(1):61–65. doi: 10.1128/jb.165.1.61-65.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakar S. N., Magee P. T. Genetic analysis of Candida albicans: identification of different isoleucine-valine, methionine, and arginine alleles by complementation. J Bacteriol. 1982 Sep;151(3):1247–1252. doi: 10.1128/jb.151.3.1247-1252.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakar S. N., Partridge R. M., Magee P. T. A genetic analysis of Candida albicans: isolation of a wide variety of auxotrophs and demonstration of linkage and complementation. Genetics. 1983 Jun;104(2):241–255. doi: 10.1093/genetics/104.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz M. B., Cortelyou M. W., Miller S. M., Lai M., Kirsch D. R. Development of autonomously replicating plasmids for Candida albicans. Mol Cell Biol. 1987 Jan;7(1):209–217. doi: 10.1128/mcb.7.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz M. B., Kirsch D. R., Kelly R. The molecular genetics of Candida albicans. Microbiol Sci. 1988 Feb;5(2):58–63. [PubMed] [Google Scholar]
- Manning M., Mitchell T. G. Analysis of cytoplasmic antigens of the yeast and mycelial phases of Candida albicans by two-dimensional electrophoresis. Infect Immun. 1980 Nov;30(2):484–495. doi: 10.1128/iai.30.2.484-495.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina M., Cenamor R., Nombela C. Exo-1,3-beta-glucanase activity in Candida albicans: effect of the yeast-to-mycelium transition. J Gen Microbiol. 1987 Mar;133(3):609–617. doi: 10.1099/00221287-133-3-609. [DOI] [PubMed] [Google Scholar]
- Nombela C., Pomés R., Gil C. Protoplasts fusion hybrids from Candida albicans morphological mutants. Crit Rev Microbiol. 1987;15(1):79–85. doi: 10.3109/10408418709104450. [DOI] [PubMed] [Google Scholar]
- Pomés R., Gil C., Nombela C. Genetic analysis of Candida albicans morphological mutants. J Gen Microbiol. 1985 Aug;131(8):2107–2113. doi: 10.1099/00221287-131-8-2107. [DOI] [PubMed] [Google Scholar]
- Ponton J., Jones J. M. Identification of two germ-tube-specific cell wall antigens of Candida albicans. Infect Immun. 1986 Dec;54(3):864–868. doi: 10.1128/iai.54.3.864-868.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncero C., Valdivieso M. H., Ribas J. C., Durán A. Effect of calcofluor white on chitin synthases from Saccharomyces cerevisiae. J Bacteriol. 1988 Apr;170(4):1945–1949. doi: 10.1128/jb.170.4.1945-1949.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd M. G. Pathogenicity of morphological and auxotrophic mutants of Candida albicans in experimental infections. Infect Immun. 1985 Nov;50(2):541–544. doi: 10.1128/iai.50.2.541-544.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd M. G., Poulter R. T., Sullivan P. A. Candida albicans: biology, genetics, and pathogenicity. Annu Rev Microbiol. 1985;39:579–614. doi: 10.1146/annurev.mi.39.100185.003051. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Herman M. A., Staebell M. A. The involvement of cell wall expansion in the two modes of mycelium formation of Candida albicans. J Gen Microbiol. 1985 Sep;131(9):2367–2375. doi: 10.1099/00221287-131-9-2367. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Mitchell L. H. Filament ring formation in the dimorphic yeast Candida albicans. J Cell Biol. 1983 Feb;96(2):486–493. doi: 10.1083/jcb.96.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R. The regulation of cellular differentiation in the dimorphic yeast Candida albicans. Bioessays. 1986 Jul;5(1):5–11. doi: 10.1002/bies.950050103. [DOI] [PubMed] [Google Scholar]
- Sundstrom P. M., Nichols E. J., Kenny G. E. Antigenic differences between mannoproteins of germ tubes and blastospores of Candida albicans. Infect Immun. 1987 Mar;55(3):616–620. doi: 10.1128/iai.55.3.616-620.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom P. M., Tam M. R., Nichols E. J., Kenny G. E. Antigenic differences in the surface mannoproteins of Candida albicans as revealed by monoclonal antibodies. Infect Immun. 1988 Mar;56(3):601–606. doi: 10.1128/iai.56.3.601-606.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Soll D. R. Mitotic recombination in Candida albicans: recessive lethal alleles linked to a gene required for methionine biosynthesis. Mol Gen Genet. 1982;187(3):477–485. doi: 10.1007/BF00332632. [DOI] [PubMed] [Google Scholar]