Abstract
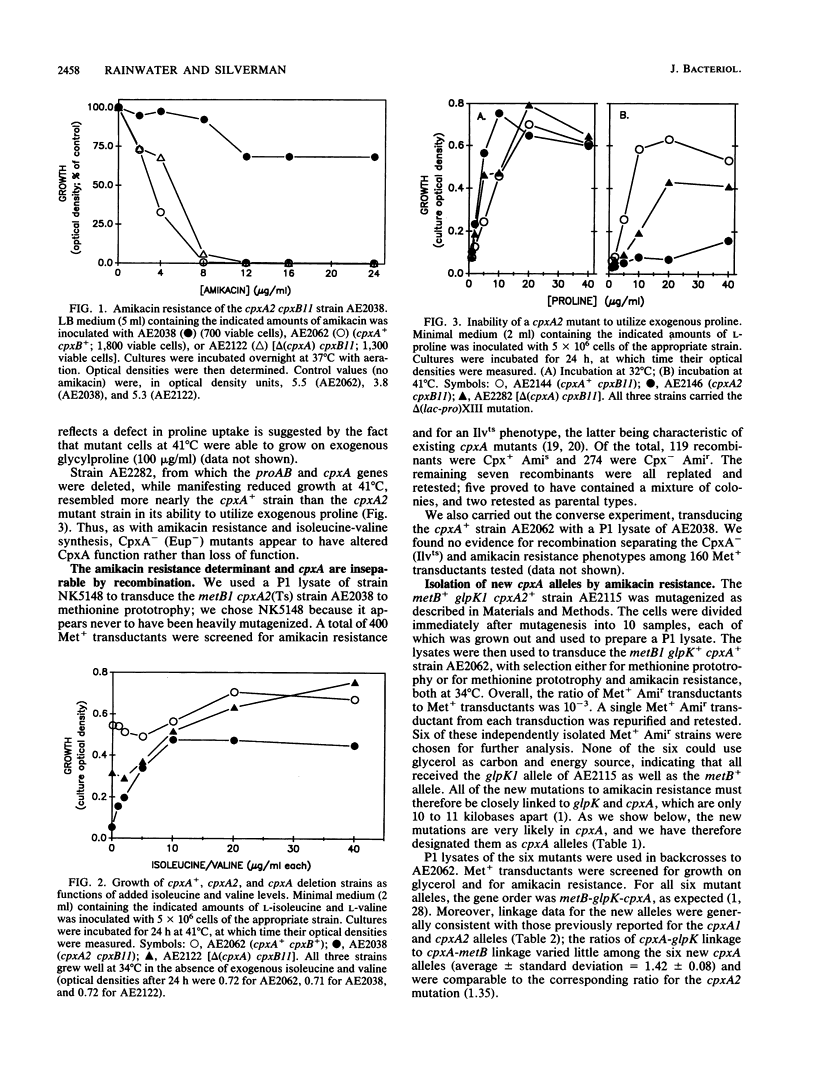
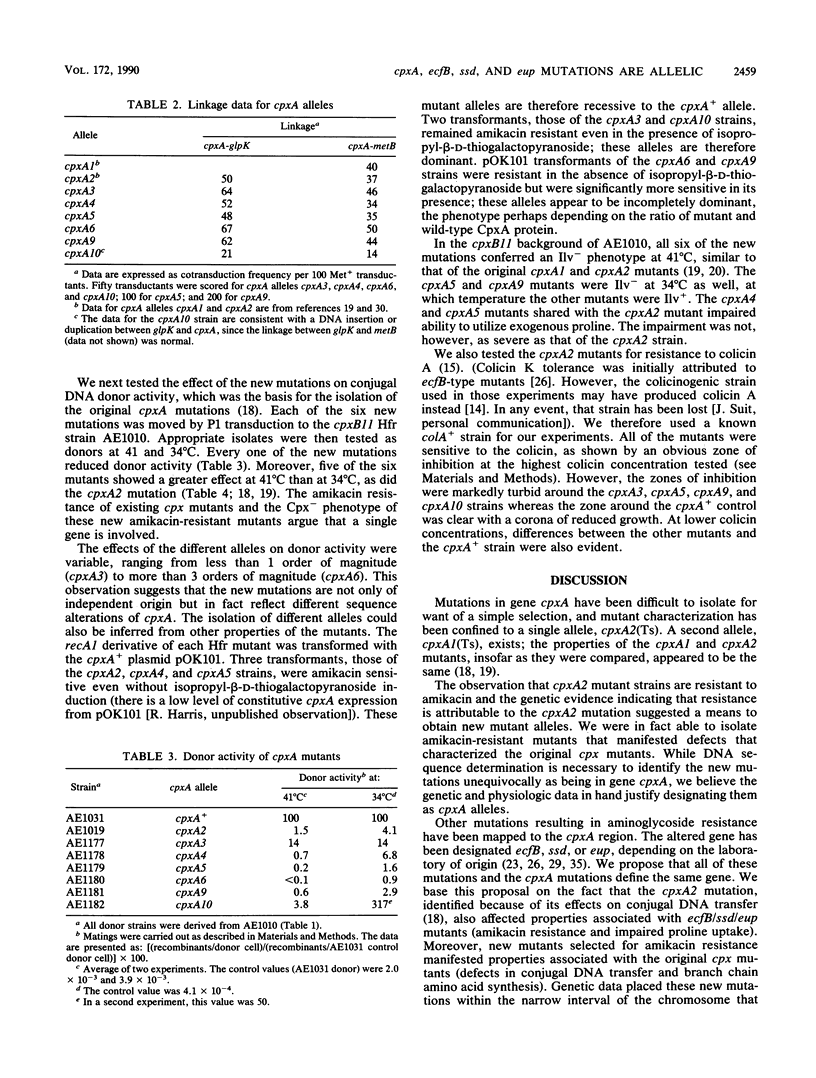
An existing cpxA(Ts) mutant was resistant to amikacin at levels that inhibited completely the growth of a cpxA+ and a cpxA deletion strain and failed to grow as efficiently on exogenous proline. These properties are similar to those of mutants altered in a gene mapped to the cpxA locus and variously designated as ecfB, ssd, and eup. The amikacin resistance phenotype of the cpxA mutant was inseparable by recombination from the cpxA mutant phenotype (inability to grow at 41 degrees C without exogenous isoleucine and valine) and was recessive to the cpxA+ allele of a recombinant plasmid. Using methods that ensured independent mutations in the cpxA region of the chromosome, we isolated six new amikacin-resistant mutants following nitrosoguanidine mutagenesis. Three-factor crosses mapped the mutations to the cpxA locus. When transferred by P1 transduction to a cpxB11 Hfr strain, each of the mutations conferred the Tra- and Ilv- phenotypes characteristic of earlier cpxA mutants. Two of the new mutations led to a significantly impaired ability to utilize exogenous proline, and four led to partial resistance to colicin A. Two of the new cpxA alleles were recessive to the cpxA+ allele, and four were dominant, albeit to different degrees. On the basis of these data, we argue that cpxA, ecfB, eup, and ssd are all the same gene. We discuss the cellular function of the cpxA gene product in that light.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albin R., Silverman P. M. Identification of the Escherichia coli K-12 cpxA locus as a single gene: construction and analysis of biologically-active cpxA gene fusions. Mol Gen Genet. 1984;197(2):272–279. doi: 10.1007/BF00330973. [DOI] [PubMed] [Google Scholar]
- Albin R., Silverman P. M. Physical and genetic structure of the glpK-cpxA interval of the Escherichia coli K-12 chromosome. Mol Gen Genet. 1984;197(2):261–271. doi: 10.1007/BF00330972. [DOI] [PubMed] [Google Scholar]
- Albin R., Weber R., Silverman P. M. The Cpx proteins of Escherichia coli K12. Immunologic detection of the chromosomal cpxA gene product. J Biol Chem. 1986 Apr 5;261(10):4698–4705. [PubMed] [Google Scholar]
- Bourret R. B., Hess J. F., Borkovich K. A., Pakula A. A., Simon M. I. Protein phosphorylation in chemotaxis and two-component regulatory systems of bacteria. J Biol Chem. 1989 May 5;264(13):7085–7088. [PubMed] [Google Scholar]
- Cuozzo M., Silverman P. M. Characterization of the F plasmid TraJ protein synthesized in F' and Hfr strains of Escherichia coli K-12. J Biol Chem. 1986 Apr 15;261(11):5175–5179. [PubMed] [Google Scholar]
- Hitchens G. D., Kell D. B., Morris J. G. Transmembrane respiration-driven H+ translocation is unimpaired in an eup mutant of Escherichia coli. J Gen Microbiol. 1982 Sep;128(9):2207–2209. doi: 10.1099/00221287-128-9-2207. [DOI] [PubMed] [Google Scholar]
- Iuchi S., Cameron D. C., Lin E. C. A second global regulator gene (arcB) mediating repression of enzymes in aerobic pathways of Escherichia coli. J Bacteriol. 1989 Feb;171(2):868–873. doi: 10.1128/jb.171.2.868-873.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Furlong D., Lin E. C. Differentiation of arcA, arcB, and cpxA mutant phenotypes of Escherichia coli by sex pilus formation and enzyme regulation. J Bacteriol. 1989 May;171(5):2889–2893. doi: 10.1128/jb.171.5.2889-2893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Effects of aerobiosis and nitrogen source on the proton motive force in growing Escherichia coli and Klebsiella pneumoniae cells. J Bacteriol. 1981 Apr;146(1):377–384. doi: 10.1128/jb.146.1.377-384.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Guffanti A. A. Alkalophilic bacteria. Annu Rev Microbiol. 1989;43:435–463. doi: 10.1146/annurev.mi.43.100189.002251. [DOI] [PubMed] [Google Scholar]
- Luria S. E. The mistaken identity of colicin A. J Bacteriol. 1982 Jan;149(1):386–386. doi: 10.1128/jb.149.1.386-386.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen J., Silverman P. M. Mutations in genes cpxA and cpxB alter the protein composition of Escherichia coli inner and outer membranes. J Bacteriol. 1982 Sep;151(3):1553–1559. doi: 10.1128/jb.151.3.1553-1559.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen J., Silverman P. Chromosomal mutations of Escherichia coli that alter expression of conjugative plasmid functions. Proc Natl Acad Sci U S A. 1980 Jan;77(1):513–517. doi: 10.1073/pnas.77.1.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen J., Silverman P. Genetic analysis of Escherichia coli K-12 chromosomal mutants defective in expression of F-plasmid functions: identification of genes cpxA and cpxB. J Bacteriol. 1980 Oct;144(1):60–67. doi: 10.1128/jb.144.1.60-67.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen J., Silverman P. Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a defect in isoleucine and valine syntheses. J Bacteriol. 1980 Oct;144(1):68–73. doi: 10.1128/jb.144.1.68-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. F., Newman E. B. Map location of the ssd mutation in Escherichia coli K-12. J Bacteriol. 1980 Sep;143(3):1504–1505. doi: 10.1128/jb.143.3.1504-1505.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S. Novel mutations affecting a signaling component for chemotaxis of Escherichia coli. J Bacteriol. 1980 Jun;142(3):953–961. doi: 10.1128/jb.142.3.953-961.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate C. A. Mutant of Escherichia coli defective in response to colicin K and in active transport. J Bacteriol. 1976 Feb;125(2):467–474. doi: 10.1128/jb.125.2.467-474.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate C. A., Seely S. A., Laffler T. G. Evidence for a protonmotive force related regulatory system in Escherichia coli and its effects on lactose transport. Biochemistry. 1986 Oct 7;25(20):6127–6132. doi: 10.1021/bi00368a044. [DOI] [PubMed] [Google Scholar]
- Plate C. A., Suit J. L. The eup genetic locus of Escherichia coli and its role in H+/solute symport. J Biol Chem. 1981 Dec 25;256(24):12974–12980. [PubMed] [Google Scholar]
- Sambucetti L., Eoyang L., Silverman P. M. Cellular control of conjugation in Escherichia coli K12. Effect of chromosomal cpx mutations on F-plasmid gene expression. J Mol Biol. 1982 Oct 15;161(1):13–31. doi: 10.1016/0022-2836(82)90275-3. [DOI] [PubMed] [Google Scholar]
- Silverman P. M. Gene cpxA is a new addition to the linkage map of Escherichia coli K-12. J Bacteriol. 1982 Apr;150(1):425–428. doi: 10.1128/jb.150.1.425-428.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H. S., Lang B. F., Newman E. B. L-serine degradation in Escherichia coli K-12: cloning and sequencing of the sdaA gene. J Bacteriol. 1989 Sep;171(9):5095–5102. doi: 10.1128/jb.171.9.5095-5102.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Newman T., McEwen J., Silverman P. M., Freundlich M. Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a defect in acetohydroxyacid synthase I function in vivo. J Bacteriol. 1982 Aug;151(2):976–982. doi: 10.1128/jb.151.2.976-982.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber H. W., Mueller J. P., Miller P. F., Arrow A. S. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev. 1987 Dec;51(4):439–457. doi: 10.1128/mr.51.4.439-457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorbjarnardóttir S. H., Magnúsdóttir R. A., Eggertsson G. Mutations determining generalized resistance to aminoglycoside antibiotics in Escherichia coli. Mol Gen Genet. 1978 Apr 25;161(1):89–98. doi: 10.1007/BF00266619. [DOI] [PubMed] [Google Scholar]
- Varenne S., Knibiehler M., Cavard D., Morlon J., Lazdunski C. Variable rate of polypeptide chain elongation for colicins A, E2 and E3. J Mol Biol. 1982 Jul 25;159(1):57–70. doi: 10.1016/0022-2836(82)90031-6. [DOI] [PubMed] [Google Scholar]
- Yamazaki H. The mechanism of early inhibition by an RNA phage of protein and RNA synthesis in infected cells. Virology. 1969 Mar;37(3):429–436. doi: 10.1016/0042-6822(69)90226-8. [DOI] [PubMed] [Google Scholar]


