Abstract
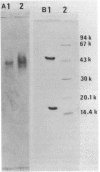
A protease that nicks the approximately 150-kilodalton (kDa) single-chain type A botulinum neurotoxin into the approximately 150-kDa di-chain form in vitro was isolated from Clostridium botulinum type A (Hall strain) cultures. The di-chain neurotoxin generated in vitro is composed of an approximately 50-kDa light chain and an approximately 100-kDa heavy chain which are disulfide linked and is indistinguishable from the di-chain neurotoxin that forms in vivo and is routinely isolated (M.L. Dekleva and B.R. DasGupta, Biochem. Biophys. Res. Commun. 162:767-772, 1989). This enzyme was purified greater than 1,000-fold by ammonium sulfate precipitation, QAE-Sephadex Q-50, Sephadex G-100, and CM-Sephadex C-50 chromatography steps with the synthetic substrate N-benzoyl-DL-arginine-p-nitroanilide. The approximately 62-kDa amidase (protease) is a complex of 15.5- and 48-kDa polypeptides (determined by polyacrylamide gel electrophoresis) that could not be separated without sodium dodecyl sulfate. The enzyme has an isoelectric point of pH 5.73, a pH optimum of 6.2 to 6.4, an absolute requirement for a thiol-reducing agent as well as a divalent metallic cation (probably Ca2+) for activity, and a temperature optimum of 70 degrees C. Tests with several synthetic substrates indicated the high specificity of the enzyme for arginyl amide bonds.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONVENTRE P. F., KEMPE L. L. Physiology of toxin production by Clostridium botulinum types A and B. I. Growth, autolysis, and toxin production. J Bacteriol. 1960 Jan;79:18–23. doi: 10.1128/jb.79.1.18-23.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta B. R. Activation of Clostridium botulinum type B toxin by an endogenous enzyme. J Bacteriol. 1971 Dec;108(3):1051–1057. doi: 10.1128/jb.108.3.1051-1057.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta B. R., Sathyamoorthy V. Purification and amino acid composition of type A botulinum neurotoxin. Toxicon. 1984;22(3):415–424. doi: 10.1016/0041-0101(84)90085-0. [DOI] [PubMed] [Google Scholar]
- Dasgupta B. R., Sugiyama H. Isolation and characterization of a protease from Clostridium botulinum type B. Biochim Biophys Acta. 1972 Jun 16;268(3):719–729. doi: 10.1016/0005-2744(72)90276-8. [DOI] [PubMed] [Google Scholar]
- Dekleva M. L., DasGupta B. R. Nicking of single chain Clostridium botulinum type A neurotoxin by an endogenous protease. Biochem Biophys Res Commun. 1989 Jul 31;162(2):767–772. doi: 10.1016/0006-291x(89)92376-0. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- KINDLER S. H., MAGER J., GROSSOWICZ N. Production of toxin by resting cells of Cl. Parabotulinum type A. Science. 1955 Nov 11;122(3176):926–927. doi: 10.1126/science.122.3176.926. [DOI] [PubMed] [Google Scholar]
- Krysinski E. P., Sugiyama H. Nature of intracellular type A botulinum neurotoxin. Appl Environ Microbiol. 1981 Mar;41(3):675–678. doi: 10.1128/aem.41.3.675-678.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell W. M., Harrington W. F. Purification and properties of clostridiopeptidase B (Clostripain). J Biol Chem. 1968 Sep 25;243(18):4683–4692. [PubMed] [Google Scholar]
- Miura T. Isolation and characterization of an esterase-active enzyme from pronase with special reference to activation of Clostridium botulinum type E progenitor toxin. Jpn J Med Sci Biol. 1974 Dec;27(6):285–296. doi: 10.7883/yoken1952.27.285. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Sakaguchi G. Response of type B and E Botulinum toxins to purified sulfhydryl-dependent protease produced by Clostridium botulinum type F. Jpn J Med Sci Biol. 1977 Aug;30(4):179–190. doi: 10.7883/yoken1952.30.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I., Okada T., Sakaguchi G. Responses of Clostridium botulinum type B and E progenitor toxins to some clostridial sulfhydryl-dependent proteases. Jpn J Med Sci Biol. 1975 Jun;28(3):157–164. doi: 10.7883/yoken1952.28.157. [DOI] [PubMed] [Google Scholar]
- Redinbaugh M. G., Turley R. B. Adaptation of the bicinchoninic acid protein assay for use with microtiter plates and sucrose gradient fractions. Anal Biochem. 1986 Mar;153(2):267–271. doi: 10.1016/0003-2697(86)90091-6. [DOI] [PubMed] [Google Scholar]
- Regnier F. E. High-performance liquid chromatography of proteins. Methods Enzymol. 1983;91:137–190. doi: 10.1016/s0076-6879(83)91016-9. [DOI] [PubMed] [Google Scholar]
- SCHWERT G. W., TAKENAKA Y. A spectrophotometric determination of trypsin and chymotrypsin. Biochim Biophys Acta. 1955 Apr;16(4):570–575. doi: 10.1016/0006-3002(55)90280-8. [DOI] [PubMed] [Google Scholar]
- Sakaguchi G. Clostridium botulinum toxins. Pharmacol Ther. 1982;19(2):165–194. doi: 10.1016/0163-7258(82)90061-4. [DOI] [PubMed] [Google Scholar]