Abstract
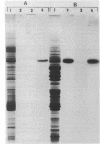
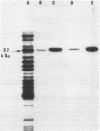
Phosphorylation of a major 57-kilodalton protein substrate was observed in cell lysates of Spiroplasma melliferum BC3 incubated with [gamma-32P]ATP. Only serine phosphates have been isolated from the acid hydrolysate of the phosphorylated protein. The 57-kilodalton protein substrate was found, to a large extent, in the cytosolic fraction and, to a lesser extent, associated with cell membranes and was detected in the Triton X-100-insoluble fraction that contained fibrils.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in the hormonal control of enzyme activity. Eur J Biochem. 1985 Sep 16;151(3):439–448. doi: 10.1111/j.1432-1033.1985.tb09121.x. [DOI] [PubMed] [Google Scholar]
- Deutscher J., Saier M. H., Jr ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6790–6794. doi: 10.1073/pnas.80.22.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M., Shirvan M. H., Platt M. W., Kirchhoff H., Rottem S. Characterization of membrane components of the flask-shaped mycoplasma Mycoplasma mobile. J Gen Microbiol. 1988 Aug;134(8):2385–2392. doi: 10.1099/00221287-134-8-2385. [DOI] [PubMed] [Google Scholar]
- Göbel U., Speth V., Bredt W. Filamentous structures in adherent Mycoplasma pneumoniae cells treated with nonionic detergents. J Cell Biol. 1981 Nov;91(2 Pt 1):537–543. doi: 10.1083/jcb.91.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Inagaki M., Gonda Y., Matsuyama M., Nishizawa K., Nishi Y., Sato C. Intermediate filament reconstitution in vitro. The role of phosphorylation on the assembly-disassembly of desmin. J Biol Chem. 1988 Apr 25;263(12):5970–5978. [PubMed] [Google Scholar]
- Kirchhoff H., Rosengarten R., Lotz W., Fischer M., Lopatta D. Flask-shaped mycoplasmas: properties and pathogenicity for man and animals. Isr J Med Sci. 1984 Sep;20(9):848–853. [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Neimark H. Mycoplasma and bacterial proteins resembling contractile proteins: a review. Yale J Biol Med. 1983 Sep-Dec;56(5-6):419–423. [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S. Protein phosphorylation in bacterial chemotaxis. Cell. 1988 Apr 8;53(1):1–2. doi: 10.1016/0092-8674(88)90478-3. [DOI] [PubMed] [Google Scholar]
- Platt M. W., Rottem S., Milner Y., Barile M. F., Peterkofsky A., Reizer J. Protein phosphorylation in Mycoplasma gallisepticum. Eur J Biochem. 1988 Sep 1;176(1):61–67. doi: 10.1111/j.1432-1033.1988.tb14251.x. [DOI] [PubMed] [Google Scholar]
- Platt M. W., Rottem S. Phosvitin kinase activity in Acholeplasma axanthum. FEBS Lett. 1988 Dec 19;242(1):97–100. doi: 10.1016/0014-5793(88)80993-1. [DOI] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Saier M. H., Jr, Deutscher J., Grenier F., Thompson J., Hengstenberg W. The phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: properties, mechanism, and regulation. Crit Rev Microbiol. 1988;15(4):297–338. doi: 10.3109/10408418809104461. [DOI] [PubMed] [Google Scholar]
- Saha A. K., Dowling J. N., Mukhopadhyay N. K., Glew R. H. Demonstration of two protein kinases in extracts of Legionella micdadei. J Gen Microbiol. 1988 May;134(5):1275–1281. doi: 10.1099/00221287-134-5-1275. [DOI] [PubMed] [Google Scholar]
- Szczesna D., Sobieszek A., Kakol I. Binding of phosphorylated and dephosphorylated heavy meromyosin to F-actin. FEBS Lett. 1987 Jan 5;210(2):177–180. doi: 10.1016/0014-5793(87)81332-7. [DOI] [PubMed] [Google Scholar]
- Townsend R., Archer D. B., Plaskitt K. A. Purification and preliminary characterization of Spiroplasma fibrils. J Bacteriol. 1980 May;142(2):694–700. doi: 10.1128/jb.142.2.694-700.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Koshland D. E., Jr Evidence for protein kinase activities in the prokaryote Salmonella typhimurium. J Biol Chem. 1978 Nov 10;253(21):7605–7608. [PubMed] [Google Scholar]
- Williamson D. L. Unusual fibrils from the spirochete-like sex ratio organism. J Bacteriol. 1974 Feb;117(2):904–906. doi: 10.1128/jb.117.2.904-906.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]